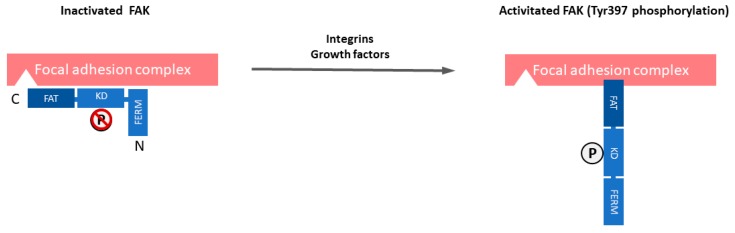
Figure 1.
The domain organization and activation of focal adhesion kinase (FAK). FAK is composed of a central kinase domain (KD), an amino-terminal side that is flanked by a protein band 4.1-ezrin-radixin-moesin (FERM) homology domain, and a carboxy-terminal focal adhesion targeting (FAT) domain flanked by proline-rich regions (PRRs). FAK localizes to focal adhesions and is triggered off by extracellular signals such as integrin-mediated adhesion and some growth factors. FAK is maintained in an inactive state by the binding of the FERM domain to the kinase domain, which blocks access to the catalytic site and sequesters the activation loop, as well as the key autophosphorylation site tyrosine 397 (Tyr397). Engagement of integrins with the extracellular matrix (ECM) or growth factors leads to signals that displace the FERM domain, resulting in rapid autophosphorylation of Tyr397, which is a critical event in signal transduction by FAK.