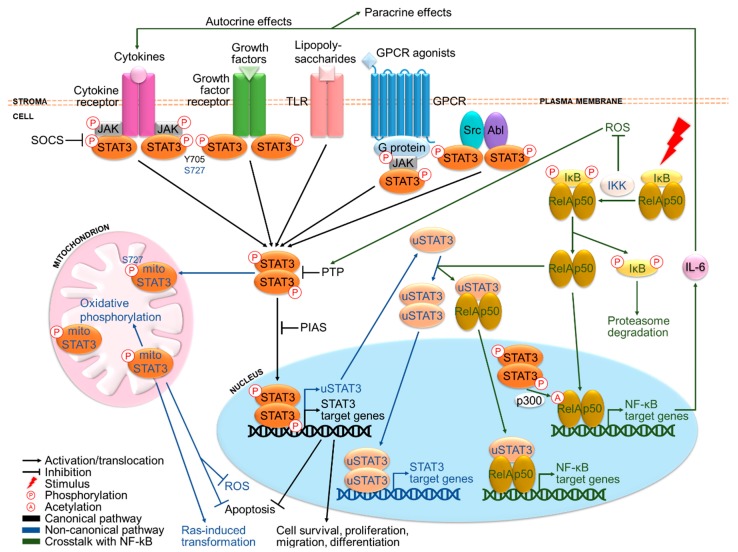
Figure 1.
The STAT3 signaling pathway and its crosstalk with NF-kB. STAT3 is activated primarily by cytokines and growth factors, in addition to other signaling molecules. Canonically, ligand binding to receptors triggers phosphorylation of tyrosine kinases and subsequently STAT3 at Y705, followed by STAT3 dimerization and translocation to the nucleus where it drives transcription of target genes involved in cell survival and proliferation. Non-canonically, STAT3 can also be phosphorylated at S727, translocate to the mitochondrion, as well as autoregulate its own transcription to produce u-STAT3. Under normal conditions, STAT3 activation is under tight negative regulation by SOCS, PTP and PIAS members. Remarkably, STAT3 is involved in extensive crosstalk with the inflammatory NF-kB pathway. Activated NF-kB has been reported to either activate or inhibit STAT3 signaling, respectively by producing various cytokines including the major STAT3-inducing cytokine IL-6 and preventing reactive oxygen species (ROS) accumulation responsible for oxidizing negative regulators of STAT3. In return, STAT3 may sustain NF-kB activation via p300-mediated acetylation. Moreover, u-STAT3 and u-NF-kB can work in concert to coregulate another set of genes.