Figure 3.
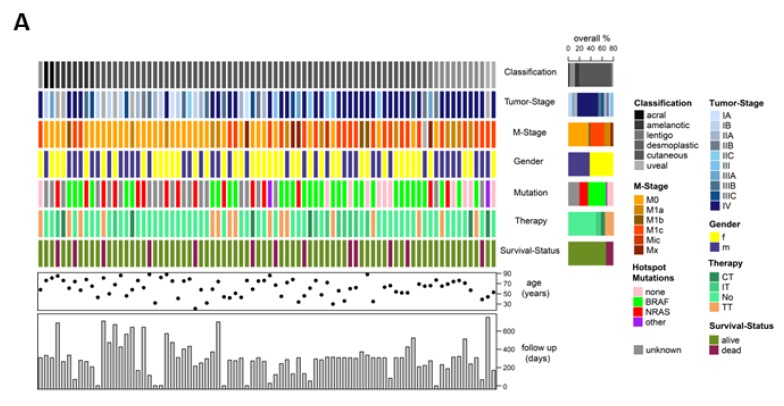
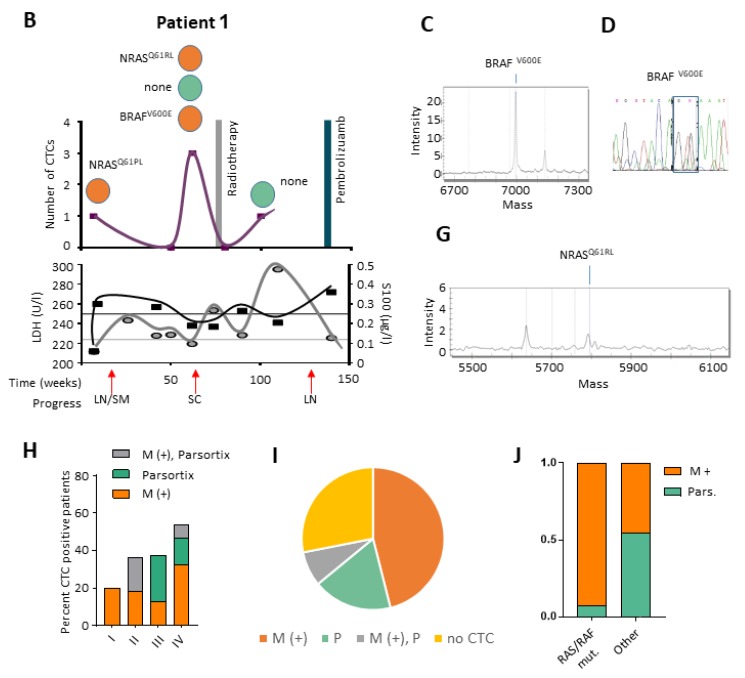
Combined enrichment method for the analysis of cellular subpopulations of circulating melanoma cells in patients. (A) Overview of patients with cutaneous, acral, mucosal and uveal melanoma of different disease stages, included in the study. (B) Time course of CTCs, enriched by positive selection (orange circles) and Parsortix™ (green circles) and their detected mutations, as well as LDH and S100 serum levels in patient #1. (C) Mass spectrum plot of BRAFV600E mutation and (D) Sanger sequencing of the same sample. (G) Mass spectrum plot of NRASQ61RL mutation. (H) Percentage of patients positive for circulating melanoma cells enriched by positive selection (MACS, M+), Parsortix™ or both. (I) Distribution of enriched CTCs in stage 4 patients prior to treatment. (J) CTCs enriched by positive selection, and Parsortix™ in NRAS/BRAF mutated patients, and not BRAF/NRAS mutated patients.