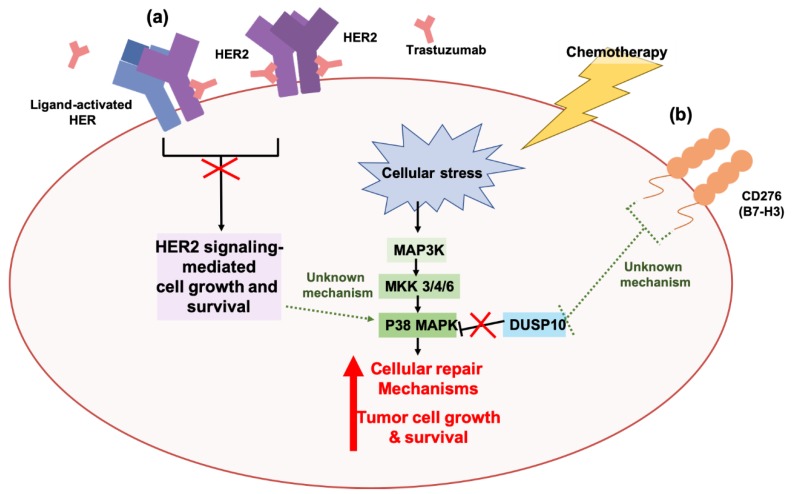
Figure 2.
The stress-activated p38 MAPK pathway contributes to tumor resistance against targeted therapies and chemotherapies in cancers. (a) Overexpression of the human epidermal growth factor receptor 2 (HER2) protein has been observed in cancers, like breast, and has been associated with the aberrant activation of growth and survival pathways, like AKT signaling and ERK signaling. The HER2-targeted antibody trastuzumab binds to the extracellular domain of HER2 and inhibits HER2 dimerization and HER2-mediated signaling events, consequently mitigating tumorigenesis. Ectopic activation of the p38 MAPK pathway in HER2-overexpressing (HER2+) breast cancers can confer resistance to trastuzumab by promoting cell growth and survival independent of HER2 activity [331]. To date, the mechanisms underlying the relationship between HER2 signaling and p38 MAPK remain unknown. (b) Resistance to chemotherapies, like cisplatin and dacarbazine, have also been associated with p38 MAPK activity in melanoma cells. Chemotherapy-induced cellular stress activates the p38 MAPK pathway, and this results in the activation of cellular repair mechanisms that promote tumor cell growth and survival. Activation of p38 MAPK is further sustained by the immune checkpoint molecule, CD276/B7-H3, which inhibits the p38 MAPK-negative regulator dual specificity protein phosphatase 10 (DUSP10) [335]. To date, the mechanistic relationship between CD276/B7-H3 activity and DUSP10 inhibition remains unknown.