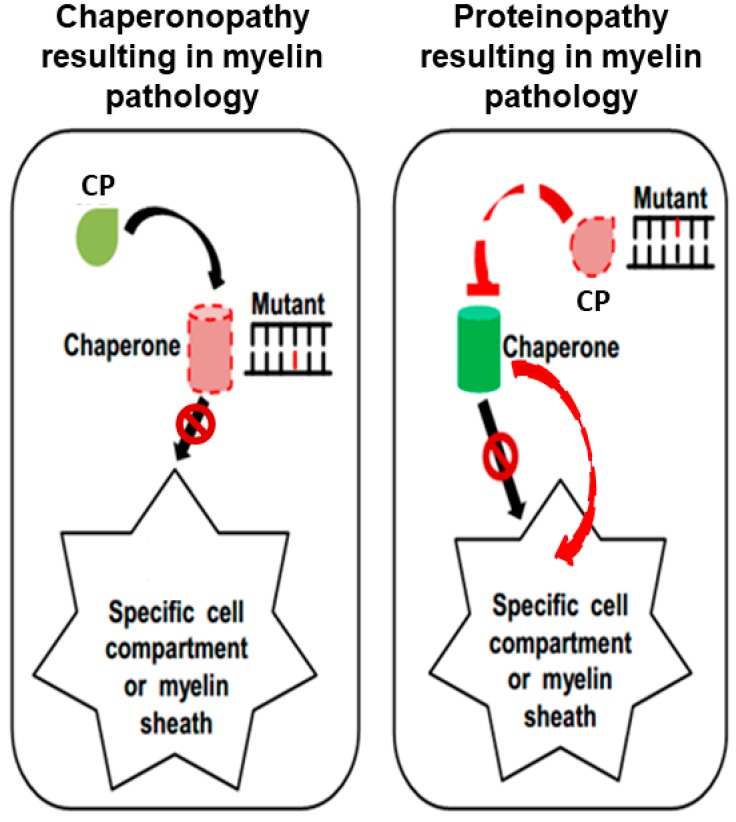
Figure 1.
Hypothetical mechanisms involving chaperones in myelination and myelin pathology. Molecular chaperones (normal: green cylinder; abnormal: pink cylinder with dashed-red borders) assist the folding of myelin proteins (drop-shaped icons; normal, light green; abnormal, pink with dashed-red border) in the cell and their migration toward the myelin sheath, and/or directly in situ in the myelin sheath. In order to perform these tasks, chaperones bind their client proteins (substrate; client myelin polypeptide (CP)). The left panel illustrates a chaperonopathy (chaperone gene mutant, indicated by Mutant), that is, a chaperone deficiency causes incorrect myelination. An abnormal chaperone is the primary cause of myelinopathy: the chaperone’s lack of function, or partial insufficiency (e.g., due to mutation in its gene) cannot correctly fold its client myelin polypeptide (CP) and/or cannot assist its migration to its functional residence (black arrow with forbidden sign). In the right panel, the myelinopathy is caused by a mutation in the gene encoding the myelin protein affected (indicated by Mutant), while the chaperone genes are normal. An indirect chaperone insufficiency may occur if the chaperone cannot bind and interact with its substrate because the latter is abnormal, and thus it cannot be recognized or bound properly by the chaperone (top red truncated arrow); or the normal chaperone can bind the defective protein substrate but cannot fold and transport it to the place in which it functions, for example, the myelin sheath (black arrow with forbidden sign); or the chaperone transports the client protein to the pertinent compartment, but the protein does not correctly function due to mutation (curved red arrow to the right).