Abstract
The PARP inhibitor olaparib has been approved in the maintenance setting of platinum-sensitive epithelial ovarian cancer patients with germline or somatic BRCA1/2 mutation. Therefore, the availability of a tumor BRCA test has become a clinical need. We report the results of the clinical implementation of a tumor BRCA test within the frame of an institutional workflow for the management of patients with nonmucinous and nonborderline epithelial ovarian cancer. In total, 223 patients with epithelial ovarian cancer were prospectively analyzed. BRCA1/2 status was evaluated on formalin-fixed, paraffin-embedded tumor specimens using next-generation sequencing technology. The tumor BRCA test had a success rate of 99.1% (221 of 223 successfully analyzed cases) and a median turnaround time of 17 calendar days. Among the 221 cases, BRCA1 or BRCA2 pathogenic/likely pathogenic mutations were found in 62 (28.1%) cases and variants of uncertain significance in 25 (11.3%) cases. The concordance rate between tumor BRCA test results and germline BRCA1/2 status was 87%, with five cases harboring pathogenic/likely pathogenic somatic-only mutations. The next-generation, sequencing-based tumor BRCA test showed a high success rate and a turnaround time compatible with clinical purposes. The tumor BRCA test could be implemented in a molecular diagnostic setting and it may guide the clinical management of patients with epithelial ovarian cancer.
Keywords: BRCA1/2, ovarian cancer, next-generation sequencing
1. Introduction
Epithelial ovarian cancer (EOC) is the seventh most common cancer among women worldwide [1], frequently diagnosed in an advanced stage and with an aggressive clinical course [2]. Despite surgical resection and standard chemotherapy, the frequency of disease relapse at 5 years may reach 40–75% [3]. The introduction of poly (adenosine diphosphate (ADP)-ribose) polymerase inhibitors (PARPi) has marked a crucial step forward in the treatment of these patients. Up until now, three PARPi—olaparib, niraparib, rucaparib—have received the Food and Drug Administrations (FDA) and European Medicines Agency (EMA) approvals in different clinical settings [4,5,6]. Olaparib has been approved in Europe for the maintenance of platinum-sensitive recurrent EOCs patients with a somatic or germline BRCA1/2 mutation [4]. Moreover, based on the recent striking results of SOLO 1 trial, olaparib will be used as maintenance therapy even for women with newly diagnosed advanced EOC harboring BRCA1/2 mutations [7]. Therefore, the evaluation of BRCA1 and BRCA2 status is nowadays mandatory for all patients with advanced nonmucinous EOC to guide the therapy decision. In this setting, the analysis of BRCA1/2 should meet precise requirements [8,9]. First, the test should be performed on tumor specimens, enabling to capture both somatic and germline alterations; second, the test should be suitable for formalin-fixed, paraffin-embedded (FFPE) specimens, available in all the pathology laboratories; third, the test turnaround time should be consistent with treatment decision purposes; finally, the test should be included in a comprehensive and multidisciplinary clinical management of women with EOC.
In the last years, different studies have reported on the implementation and validation of next-generation sequencing (NGS) platforms to assess the BRCA1/2 status in FFPE specimens [10,11,12]. Although these NGS panels allowed a shorter test turnaround time (TAT) as compared with standard Sanger sequencing, the use of these assays in a clinical diagnostic scenario may be challenging [8,9].
In this study, we prospectively analyzed the tumor BRCA1/2 status in FFPE samples from 223 patients with EOC, aiming (i) to ascertain the feasibility of the tumor NGS-based BRCA test in the clinical setting, and (ii) to evaluate the prevalence of tumor BRCA1 and BRCA2 mutations in a single-institution cohort of women with epithelial ovarian cancers.
2. Results
2.1. Tumor BRCA Test Performance
All the FFPE tumor samples were considered adequate for the analysis according to the tumor cell content (more than 10%) and the DNA yield (more than 10 ng). In two cases (0.9%), the NGS analysis failed due to low NGS run quality parameters and FFPE artifacts. Overall, the median TAT was 17 calendar days (range 4–50 calendar days) from test request to final molecular report. During the timeframe of the analysis (25 months), the number of test demands had increased, in association with a decrease in TAT. In the first trimester, 12 requests had been handled with a median TAT of 33 calendar days, in the last trimester, 46 requests had been processed with a median TAT of 14 calendar days.
2.2. Tumor BRCA1 and BRCA2 Alterations Distribution
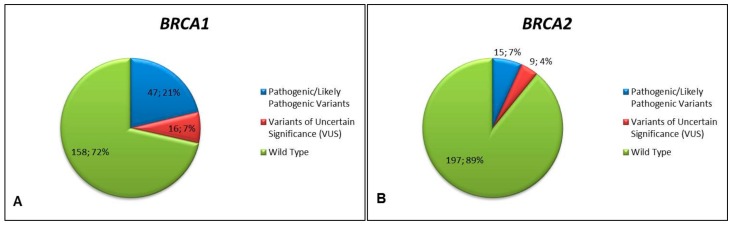
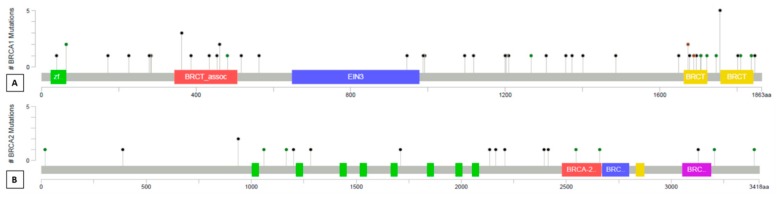
Among the 221 cases successfully analyzed, 62 (28.1%) cases harbored tumor BRCA1 or BRCA2 pathogenic/likely pathogenic mutations. Twenty-five (11.3%) cases harbored a variant of uncertain significance (VUS), including three cases with BRCA2 VUS and a concurrent BRCA1 pathogenic mutation. No other co-occurrence of pathogenic or likely pathogenic mutations was observed. Overall, 87 variants were identified (BRCA1 n = 63, 72.4%; BRCA2 n = 24, 27.6%), with a median coverage of 1987 reads and a median variant allele frequency of 70%. In detail, 47 pathogenic/likely pathogenic mutations and 16 VUS were identified in BRCA1; 15 pathogenic/likely pathogenic mutations and nine VUS occurred in BRCA2 (Figure 1). The mutations distribution spanned the whole coding sequence of BRCA1 and BRCA2 gene, without hotspot regions (Figure 2). Eleven and five alterations affecting the intronic regions were identified in BRCA1 and BRCA2, respectively.
Figure 1.
Tumor BRCA1 (A) and BRCA2 (B) status in the study cohort according to variant classification. Pie charts representing the distribution (number of cases; percentage) of BRCA1 (A) and BRCA2 (B) variants among the 221 successfully analyzed. Variant classification is color-coded according to the legend on the right.
Figure 2.
Lollipop plots of mutation distribution on BRCA1 (A) and BRCA2 (B) protein and domains. The diagrams linearly represent BRCA1/2 protein domains (x-axis). BRCA1 domains: green, C3HC4-type RING finger; red, serine-rich domain associated with BRCT; blue, ethylene insensitive 3; yellow, BRCA1 C terminus domain. BRCA2 domains: green, BRCA2 repeats; red, BRCA2 helical; blue, BRCA2 oligonucleotide/oligosaccharide-binding domain 1; yellow, tower; purple, BRCA2 oligonucleotide/oligosaccharide-binding domain 3. Each mutation is represented by single lollipop; the stick lengths indicate mutation frequency (y-axis), and dots are color-coded according to alteration type: green, missense mutations; black, truncating mutations (frameshift or nonsense mutations); brown dots, in-frame mutations. Intronic variants are not reported. Graphs created using Mutation Mapper tool, cBioportal (http://www.cbioportal.org/mutation_mapper) and manually curated. BRCA1: RefSeq: NM_007294, Ensembl: ENST00000357654, CCDS: CCDS11453, UniProt: BRCA1_HUMAN. BRCA2: RefSeq: NM_000059, Ensembl: ENST00000380152, CCDS: CCDS9344, UniProt: BRCA2_HUMAN.
2.3. Tumor BRCA1/2 Status According to Clinicopathological Characteristics
The BRCA1 and BRCA2 mutations prevalence according to clinicopathological parameters was reported in Table 1. The majority of BRCA1/2 alterations were identified in primary tumors, whereas only two clinically significant mutations were identified in the sample from the recurrence site. In the high-grade ovarian serous carcinoma subgroup, 31.4% of cases harbored a clinically significant mutation (n = 61 cases, 47 BRCA1 and n = 14 BRCA2), whereas only one likely pathogenic BRCA2 alteration was detected in non-high-grade serous carcinoma. Actually, the histotype was the only clinicopathological parameter showing statistically significant association with the presence of BRCA1/2 mutation (p-value = 0.005) (Table 2). However, a higher frequency of BRCA1/2 mutations was identified among patients with the tumor BRCA test requested at least 6 months after diagnosis without disease-relapse (34.3%) as compared with patients with the test performed at diagnosis or disease relapse (29.3% and 16.2%, respectively). Eleven women with a positive family history previously underwent surgery for breast cancers, and in five cases BRCA1 (n = 4) or BRCA2 (n = 1), pathogenic alterations were detected in the ovary tumor samples.
Table 1.
Tumor BRCA1 and BRCA2 alterations distribution according to the clinicopathological parameters of the 221 cases successfully analyzed.
| Clinicopathological Features | BRCA1 | BRCA2 | ||||
|---|---|---|---|---|---|---|
| Pathogenic/Likely Pathogenic Mutations | VUS | WT | Pathogenic/Likely Pathogenic Mutations | VUS | WT | |
| Tumor Site | ||||||
| Primary tumors (n = 200) | 46 (23.0%) | 13 (6.5%) | 141 (70.5%) | 14 (7.0%) | 7 (3.5%) | 179 (89.5%) |
| Metastases/Recurrences (n = 21) |
1 (4.8%) | 3 (14.3%) | 17 (81.0%) | 1 (4.8%) | 2 (9.5%) | 18 (85.7%) |
| Histologycal Subtype | ||||||
| High-grade serous carcinoma (n = 194) | 47 (24.2%) | 16 (8.2%) | 131 (67.5%) | 14 (7.2%) | 7 (3.6%) | 173 (89.2%) |
| Non-serous carcinoma/subtype not defined (n = 27) | 0 | 0 | 27 (100%) | 1 (3.7%) | 2 (7.4%) | 24 (88.9%) |
| Pathological Staging | ||||||
| T1 (n = 15) | 2 (13.3%) | 0 | 13 (86.7%) | 2 (13.3%) | 2 (13.3%) | 11 (73.3%) |
| T2 (n = 29) | 6 (20.7%) | 1 (3.4%) | 22 (75.9%) | 3 (10.3%) | 0 | 26 (89.7%) |
| T3 (n = 116) | 25 (21.6%) | 8 (6.9%) | 83 (71.6%) | 4 (3.4%) | 3 (2.6%) | 109 (94.0%) |
| NA (n = 61) | 14 (23.0%) | 7 (11.5%) | 40 (65.6%) | 6 (9.8%) | 4 (6.6%) | 51 (83.6%) |
| N0 (n = 39) | 5 (12.8%) | 0 | 34 (87.2%) | 3 (7.7%) | 4 (10.3%) | 32 (82.1%) |
| N1 (n = 65) | 18 (2.7%) | 6 (9.2%) | 41 (63.1%) | 2 (3.1%) | 1 (1.5%) | 62 (95.4%) |
| NX (n = 56) | 10 (17.9%) | 3 (5.4%) | 43 (76.8%) | 4 (7.1%) | 0 | 52 (92.9%) |
| NA (n = 61) | 14 (23.0%) | 7 (11.5%) | 40 (65.6%) | 6 (9.8%) | 4 (6.6%) | 51 (83.6%) |
| Family History | ||||||
| Positive (n = 93) | 24 (25.8%) | 8 (8.6%) | 61 (65.6%) | 7 (7.5%) | 6 (6.5%) | 80 (86.0%) |
| Negative (n = 101) | 16 (15.8%) | 7 (6.9%) | 78 (77.2%) | 7 (6.9%) | 2 (2.0%) | 92 (91.1%) |
| NA (n = 27) | 7 (25.9%) | 1 (3.7%) | 19 (70.4%) | 1 (3.7%) | 1 (3.7%) | 25 (92.6%) |
| Time of Test Request | ||||||
| At pathological diagnosis (n = 123) |
28 (22.8%) | 8 (6.5%) | 87 (70.7%) | 8 (6.5%) | 5 (4.1%) | 110 (89.4%) |
| At least 6 months after pathological diagnosis without relapse (n = 35) | 9 (25.7%) | 2 (5.7%) | 24 (68.6%) | 3 (8.6%) | 3 (8.6%) | 29 (82.9%) |
| At relapse (n = 37) | 4 (10.8%) | 5 (13.5%) | 28 (75.7%) | 2 (5.4%) | 1 (2.7%) | 34 (91.9%) |
| NA (n = 26) | 6 (23.1%) | 1 (3.8%) | 19 (73.1%) | 2 (7.7%) | 0 | 24 (92.3%) |
The bold terms referred to the clinicopathological feature investigated.
Table 2.
Correlation between BRCA status and clinicopathological features.
| Clinicopathological Features | BRCA MUTATED (Phatogenic/Likely Pathogenic Mutations) | BRCA WT * | p Value ** |
|---|---|---|---|
| Tumor Site | |||
| Primary tumors (n = 200) | 62 (31.0%) | 138 (69.0%) | 0.07 |
| Metastases/Recurrences (n = 21) | 2 (9.5%) | 19 (90.5%) | |
| Histologycal Subtype | |||
| High-grade serous carcinoma (n = 194) | 61 (31.4%) | 133 (68.6%) | 0.005 |
| Non-serous carcinoma/subtype not defined (n = 27) | 1 (3.7%) | 26 (96.3%) | |
| Pathological Staging | |||
| T1 (n = 15) | 4 (26.7%) | 11 (73.3%) | 0.716 |
| T2 (n = 29) | 9 (31.0%) | 20 (69.0%) | |
| T3 (n = 116) | 29 (25.0%) | 87 (75.0%) | |
| NA (n = 61) | 20 (32.8%) | 41 (67.2%) | |
| N0 (n = 39) | 8 (20.5%) | 31 (79.5%) | 0.518 |
| N1 (n = 65) | 20 (30.8%) | 45 (69.2%) | |
| NX (n = 56) | 14 (25.0%) | 42 (75.0%) | |
| NA (n = 61) | 20 (32.8%) | 41 (67.2%) | |
| Family History | |||
| Positive (n = 93) | 31 (33.3%) | 62 (66.7%) | 0.258 |
| Negative (n = 101) | 23 (22.8%) | 78 (77.2%) | |
| NA (n = 27) | 8 (29.6%) | 19 (70.4%) | |
| Time of Test Request | |||
| At pathological diagnosis (n = 123) | 36 (29.3%) | 87 (70.7%) | 0.33 |
| At least 6 months after pathological diagnosis without relapse (n = 35) | 12 (34.3%) | 23 (65.7%) | |
| At relapse (n = 37) | 6 (16.2%) | 31 (83.8%) | |
| NA (n = 26) | 8 (30.8%) | 18 (69.2%) | |
* Wild Type (WT) or variants of uncertain significance (VUS) variants. ** p-value < 0.05, statistically significant Chi-Squared Test. The bold terms referred to the clinicopathological feature investigated.
2.4. Tumor and Germline BRCA Tests Concordance
Germline BRCA1/2 status was available for 62 patients, including 28 cases with tumor BRCA status positive (23 pathogenic/likely pathogenic alterations and five VUS). The tumor and germline BRCA test results are reported in Table 3. Overall, the concordance between tumor and germline BRCA tests was 87.1% (54 of 62), and the negative predictive value of the tumor test was 100%, as all the negative tumor cases had a negative germline test result. All the discordant cases were re-tested in an independent analysis: the specimens were re-evaluated and if available, a different sample was used for the re-test. In all the cases, the tumor BRCA results were confirmed. Five pathogenic/likely pathogenic alterations (BRCA1 n = 4 and BRCA2 n = 1) and three VUS in BRCA1 were detected in the tumor specimens only and were considered somatic mutations.
Table 3.
Correlations between tumor and germline BRCA1/2 status (n = 62).
| Tumor BRCA Test | Germline BRCA Test | |||
|---|---|---|---|---|
| Positive | Negative | |||
| Pathogenic/Likely Pathogenic | VUS | |||
| Positive | Pathogenic/Likely Pathogenic | 18 (78.3%) | / | 5 (21.7%) |
| VUS | / | 2 (40%) | 3 (60%) | |
| Negative | / | / | 34 (100%) | |
2.5. Tumor BRCA Test Workflow in Clinical Diagnostic Setting
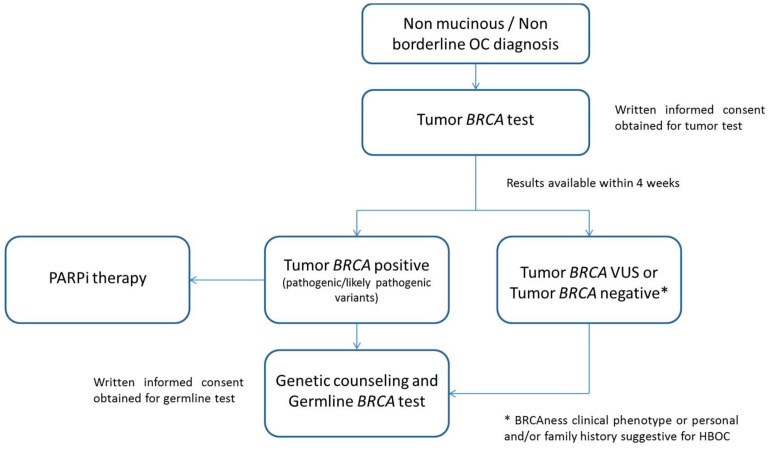
In the present study, we proposed a workflow for the analysis of tumor BRCA1/2 status in all patients with a diagnosis of nonmucinous and nonborderline EOC, according to the recently updated AIOM (Associazione Italiana di Oncologia Medica)/SIGU (Società Italiana di Genetica Umana) /SIBIOC (Società Italiana di Biochimica Clinica e Biologia Molecolare Clinica )/SIAPEC-IAP (Patologica e Citologia Diagnostica–italian division of International Academy of Pathology) guidelines [13] (Figure 3). Once the pathological report confirms the diagnosis, the gynecologic oncologist requests the tumor BRCA test upon discussion with the patients and written information on the potential test results and implications. The specific informed consent is collected by the clinician and forwarded with the BRCA test request to the Molecular Diagnostic Unit, where the tumor BRCA test is performed. The result is validated by the molecular biologist and pathologist in a timeframe expected of 4 weeks or less. The patient receives and discusses the test result with the gynecologic oncologist. Patients harboring pathogenic/likely pathogenic tumor BRCA1/2 mutations may be eligible for PARPi therapy, according to clinical characteristics, and directed to genetic counseling, during which a peripheral blood sample will be tested to evaluate the germline nature of the alteration identified. In the presence of a germline variant, prevention measures and the extension of the genetic counseling to the family members is proposed, according to the institutional procedures. Genetic counseling is advised even for patients harboring variants of unknown significance in the tumor or with a negative tumor test result if they have a suggestive clinical phenotype such as a BRCAness clinical phenotype [14] or their family history is suspicious for hereditary breast and ovarian cancer (HBOC) syndrome. For these patients, the geneticist evaluates the appropriateness of germline BRCA testing as sequencing of BRCA1/BRCA2, including the evaluation of large rearrangements or the complementary test seeking for large rearrangements only.
Figure 3.
Tumor BRCA test workflow. OC: ovarian cancer; PARPi: poly (adenosine diphosphate (ADP)-ribose) polymerase inhibitors; HBOC: hereditary breast and ovarian cancer.
3. Discussion
To date in Italy, the PARPi olaparib is approved by Agenzia Italiana del Farmaco (AIFA) as maintenance treatment after a complete or partial response to platinum-based chemotherapy for patients with recurrent high-grade serous ovarian carcinoma, carcinoma of the fallopian tube, or primary peritoneal carcinoma, with germline or somatic BRCA1/2 mutation [15]. In addition, the results of SOLO1 trial in women with newly diagnosed advanced EOC and a BRCA1/2 mutation may lead to BRCA testing at the time of diagnosis [7]. Hence, the introduction of a diagnostic test able to identify both germline and somatic BRCA1/2 alterations in tumor specimens has become necessary to guide treatment choice, even right after the surgery. In this study, we showed the clinical feasibility of an NGS-based tumor BRCA test and we proposed a BRCA test workflow to improve the clinical management of women with EOC.
There are critical pre-analytical and analytical issues in the clinical implementation of an NGS-based BRCA tumor test that are related to (i) the nature of FFPE archival specimens and (ii) the interpretation of NGS results, including mutation identification and classification [8,9,16]. Previous studies have shown that next-generation sequencing analyses represent robust and efficient methods for the detection of BRCA1/2 alterations from FFPE tumor tissue, including ovarian cancer specimen [10,11,12]. In particular, two studies validated the use of the NGS panel “Oncomine BRCA Research Assay” for tumor BRCA testing, performed on FFPE samples from ovarian cancers [12,17]. Here, we demonstrated the feasibility of the implementation of the NGS-based BRCA tumor tests in a clinical molecular diagnostic setting. All the FFPE samples met the criteria for NGS analysis, with a BRCA tumor test success rate of 99.1%. The technology was robust and fully automatized. Given that at least eight samples have to be analyzed at the same time, with increasing requests of the tumor BRCA test, the TAT from request to results has been reduced to a median of 17 calendar days. This timeframe was suitable for clinical purposes, considering both relapse treatment and maintenance therapy in newly diagnosed cancers.
In our study, 28.1% and 11.3% of cases harbored BRCA1/2 pathogenic/likely pathogenic alterations and VUS, respectively. The mutations distribution in BRCA1 and BRCA2 genes was similar to the data obtained from larger cohorts [18,19]. No hotspot mutations have been identified, as expected in a study population originating from all over Italy. We confirmed the association between the BRCA1/2 positive status and high-grade serous carcinoma histological subtype. The frequency of BRCA1/2 mutations was higher in patients with the tumor test requested at least 6 months after diagnosis without disease-relapse. Although not statistically significant, this difference may be related to the favorable prognosis and platinum-sensitivity of tumor with BRCA1/2 mutations [18]. No other clinical correlation could be evaluated due to the small number of the cases with BRCA1/2 mutation and an appropriate follow-up.
Compared with germline BRCA1/2 variant testing, the analysis on tumor tissue allow to identify also somatic alterations. These mutations account for a not insignificant proportion of BRCA1/2 mutated EOC (3.5–39%), depending on patient selection and histological subtype [18,19,20,21,22]. In the study cohort, considering the subgroup of patients with an available germline test, we detected a quota of potential somatic alterations occurring during the tumor development. In detail, 5/23 (21.7%) pathogenic/likely pathogenic mutations were identified in tumor only. These alterations would not be pinpointed if only a germline blood-based BRCA test was performed. Moreover, as recently reported by Janice Kwon, tumor BRCA testing may represent a cost-effective method of triaging women with EOC for genetic counseling and a confirmatory germline test to identify BRCA1/2 mutations carriers [23].
We proposed a BRCA test workflow for women with EOC that involved pathologists, biologists, oncologists, surgeons, and geneticists. The aim was first to offer to the patients personalized therapies both in relapse setting and in first-line maintenance treatment. Secondarily, a primary tumor BRCA screening would hasten the germline test and so accelerate the genetic counseling process, for specific cases. Indeed, for patients with BRCA-positive tumor, the germline tests could be at first instance an assessment in the peripheral blood of the alterations identified in the tumor. Thus, the germline or somatic nature of the alteration would be ascertained with different burdens, in terms of genetic counseling. In this workflow, every patient with a diagnosis of nonmucinous and nonborderline EOC would be tested. This may allow the identification of patients with a BRCA1/2 germline variant even in the absence of a family history suspicious for HBOC, that otherwise would never be included in germline BRCA screening. In the systemic review, Arts-de Jong et al. reported a 6.2% mean probability of finding a germline BRCA1/2 mutation in a patient with ovarian cancer and without a positive family history for breast and/or ovarian cancer [24]. In the present study, 23 patients with a negative family history had a BRCA tumor test positive for pathogenic/likely pathogenic alteration. Among these, four were confirmed as germline variant by a peripheral blood sample test.
The workflow we described could be a “place to start” for clinical management of women with EOC. Nevertheless, even if the negative predictive value of the tumor BRCA test for identifying germline variants was 100%, it is undisputed that the clinicians should individually evaluate each patient. First, the potential limitations of the tumor test should be considered, including the issue of accurate identification of large rearrangements as well as the tumor heterogeneity associated with the neoplastic growth [25,26]. Indeed, in our series, one case showed a BRCA1/2 negative status in the primary tumor and a subsequent BRCA1/2 positive relapse. Second, different studies reported on the phenomenon of “revertant phenotype”. Although it was not observed in our series, this phenomenon cannot be excluded a priori [27,28,29]. Third, we could analyze the concordance between tumor and germline BRCA tests only in a small subgroup of patients (n = 62/221). For these reasons, we cautiously suggest performing genetic counseling even when the tumor test is negative but the family history is suggestive for BRCAness phenotype or the patients had a personal and/or family history suggestive for HBOC syndrome.
The management of uncertain results of tumor tests remains controversial. Specific guidelines are needed for the correct handling of these results by both the gynecologic oncologist for therapeutic purposes and the clinical geneticist for genetic risk assessment. In our view, patients should be informed about the need for updates regarding the possibility of reclassification of variants of unknown significance over time.
This study has potential limitations. First, the NGS BRCA panel is currently unsuitable for a reliable measurement of copy number variants in FFPE specimens (large rearrangements accounting for less than 10% of BRCA1/2 alterations). At the present time, this pitfall is linked to the PCR enrichment methods and bioinformatics algorithms that may overcome this limit are expected. Second, a higher rate of base call error, especially in the homopolymeric region has been reported with the amplicon-based and semiconductor NGS technology as compared with other platforms [30,31,32]. However, in their validation cohort of 43 samples previously analyzed with Illumina platform and/or by Sanger sequencing, Shin et al. did not identify any false-positive variant calls in the homopolymer region, considering an allele frequency of ≥20% [17]. The authors concluded that the new Ion Torrent sequencer with an optimize bioinformatics pipeline may represent an accurate platform for the analysis of BRCA1/2 genes [17]. Furthermore, to improve the quality of variant call and reduce the error rate, we performed every sequencing run in duplicate on different chips. Third, given the short follow-up time and the small number of patients with BRCA1/2 mutations, we were not able to evaluate any correlation between BRCA1/2 status and clinical outcomes. Further studies with larger cohorts and adequate follow-up are required to assess the clinical impact of our BRCA workflow. Finally, we have not investigate the presence of alterations in other genes of the homologous recombination system. Although this evaluation may have an important clinical impact, we focused on BRCA1/BRCA2 analysis according to the current standard of care requested for predictive purpose.
4. Materials and Methods
4.1. Study Population
The study population included 223 patients with EOC referred to the Molecular Diagnostics Unit of the European Institute of Oncology from October 2016 to October 2018 for tumor BRCA1/2 analysis. The tumor BRCA test was required by gynecologic oncologists after the diagnosis of nonmucinous and nonborderline EOC, according to the test guidelines [13]. Each patient gave written informed consent to the tumor BRCA test, form institutional approved and encoded as GNM.MO.5936.B, revised version 23.10.2017.
The clinicopathological characteristics of the study cohort are summarized in Table 4. The clinical data were collected from medical records. The histological subtype and TNM staging were determined by gynecologic pathologists according to the current World Health Organization (WHO) classification of tumors of the female reproductive organs and the 8th Edition American Joint Committee on Cancer (AJCC) Staging Manual [33,34]. The family history was considered positive when at least one first degree relative had a BRCA1- or BRCA2-related cancer diagnosis. The test request was submitted immediately after the histopathological diagnosis for 123 patients, after 6 months or more from diagnosis for 35 patients without disease relapse, and at the relapse for 37 patients. For 28 patients, these data were not available. For a subgroup of patients (n = 62), germline BRCA1/2 status was also available, as previously assessed with peripheral blood testing.
Table 4.
Clinicopathological characteristics of the study population.
| Clinicopathological Features | n (%) |
|---|---|
| Time of Test Request | |
| At pathological diagnosis | 123 (55.2%) |
| At least 6 months after pathological diagnosis without relapse | 35 (15.7%) |
| At relapse | 37 (16.6%) |
| NA | 28 (12.6%) |
| Histological Subtype | |
| High-grade serous carcinoma | 195 (87.4%) |
| Endometroid carcinoma | 16 (7.2%) |
| Clear cell carcinoma | 9 (4%) |
| Carcinoma—subtype not defined | 3 (1.3%) |
| TN Pathological Staging | |
| T1 | 15 (6.7%) |
| T2 | 29 (13%) |
| T3 | 116 (52%) |
| NA | 63 (28.3%) |
| N0 | 39 (17.5%) |
| N1 | 65 (29.1%) |
| NX | 56 (25.1%) |
| NA | 63 (28.3%) |
| Specimen Analyzed | |
| Primary Tumor | 201 (90.1%) |
| Ovary localization | 169 (75.8%) |
| Extra-ovary localization | 32 (14.3%) |
| Pelvic localization | 14 (43.8%) |
| Extra-Pelvic localization | 12 (37.5%) |
| NA | 6 (18.7%) |
| Metastasis/recurrence | 22 (9.9%) |
| Lymph nodal metastasis | 3 (13.6%) |
| Extra lymph nodal metastasis | 19 (86.4%) |
| Neoadjuvant Treatment | |
| Yes | 46 (20.6%) |
| No | 114 (51.1%) |
| NA | 63 (28.3%) |
| Surgery | |
| R0 - no residual disease | 135 (60.5%) |
| R+ < 1 cm | 42 (18.8%) |
| R+ > 1 cm | 19 (8.5%) |
| NA | 27 (12.1%) |
| Family History | |
| Positive | 93 (41.7%) |
| Negative | 101 (45.3%) |
| NA | 29 (13%) |
The bold terms referred to the clinicopathological features investigated.
4.2. Next-Generation Sequencing Analysis of BRCA Status in Tumor Samples
DNA was extracted from representative formalin-fixed paraffin-embedded (FFPE) tumor tissues block as previously reported [35]. One hundred and ninety-two (86.1%) cases had FFPE tissue blocks internally stored, whereas for 31 (13.9%) cases, FFPE material was obtained from external institutions. In 134 cases (60.1%), manual macrodissection with a sterile scalpel was performed before nucleic acid isolation. The median tumor cell content was 60% (range 10–90%). BRCA1/2 status was evaluated using the next-generation sequencing (NGS) panel “Oncomine BRCA Research Assay” (ThermoFisher, Waltham, MA, USA), following the manufacturer’s instructions. Briefly, 10 ng of genomic DNA was used for the library preparation, and the subsequent chip loading performed automatically on the Ion Chef System (ThermoFisher). The sequencing run was done in duplicate using Ion S5 System (ThermoFisher). Data were analyzed using the Ion Reporter Analysis Software. Only mutations with an allele frequency ≥5% and with adequate quality metrics were reported [36]. Each clinically relevant alteration was visually inspected using the Integrative Genomics Viewer (IGV) software (Broad Institute and the Regents of the University of California, Cambridge, MA, USA). No specific algorithm for the detection of large insertion/deletion was available for FFPE tissue analysis, therefore, no copy number variant (CNV) was reported. The mutations were classified in a five-class system according to the IARC (International Agency for Research on Cancer) clinical classification and depending on the ENIGMA consortium revision [37]. The BRCA Exchange database was consulted for variant classification [38]. If the variant clinical significance was reported as “not yet reviewed” by ENIGMA consortium, other public databases were questioned, such as ClinVar [39] or Leiden Open source Variation Database (LOVD) [40]. The analytical workflow of this tumor BRCA test was previously validated on retrospective cases with known BRCA1/2 status under an inter-laboratory validation program.
4.3. Statistical Analysis
Statistical analysis was carried out using SPSS Statistic 25 software (IBM, New York, NY, USA). Chi-Square test with Yates correction was used for data comparison of categorical variables. p-values < 0.05 were considered statistically significant.
5. Conclusions
In conclusion, we showed that the NGS-based tumor BRCA test may be used in the molecular diagnostic setting for all patients with nonmucinous and nonborderline EOC. The test is suitable for the analysis of FFPE specimens and generated reliable results in a timeframe compatible with clinical needs. We proposed a “BRCA test workflow” that we followed for an integrated clinical and diagnostic management of patients with EOC, in which the tumor BRCA test may act as a compelling tool to drive a personalized treatment of these women.
Acknowledgments
The authors acknowledge laboratory technicians of the Division of Pathology, European Institute of Oncology, Milan, Italy.
Author Contributions
Conceptualization: C.F., M.B., E.G.-R.; methodology: C.F., F.T., M.B., E.G.-R.; formal analysis, C.F., F.T., I.B., A.R., L.B.; data curation, C.F., F.T., I.B., M.C., F.P., E.G.-R.; writing—original draft preparation, C.F., F.T., E.G.-R.; writing—review and editing, C.F., F.T., I.B., A.R., M.C., B.B., L.B., F.P., N.C., G.V., M.B., E.G.-R.; supervision, C.F, M.B., E.G.-R.
Funding
This research received no external funding.
Conflicts of Interest
E.G.-R. received honoraria/advisory fee from Thermofisher Scientific, Roche, Novartis, AstraZeneca, M.B. received honoraria from Thermofisher Scientific, Roche, BMS, MSD, Biocartis.
References
- 1.SEER Cancer Statistics Factsheets: Ovary Cancer. [(accessed on 11 February 2019)]; National Cancer Institute. Bethesda. Available online: https://seer.cancer.gov/statfacts/html/ovary.html.
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Du Bois A., Reuss A., Pujade-Lauraine E., Harter P., Ray-Coquard I., Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 4.AstraZeneca Lynparza Summary of Product Characteristics 2014. [(accessed on 28 June 2019)]; Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/003726/WC500180151.pdf.
- 5.Tesaro Zejula Summary of Product Characteristics 2017. [(accessed on 28 June 2019)]; Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/004249/WC500239289.pdf.
- 6.Clovis Oncology UK Rubraca Summary of Product Characteristics 2018. [(accessed on 28 June 2019)]; Available online: https://www.ema.europa.eu/documents/product-information/rubraca-epar-product-information_en.pdf.
- 7.Moore K., Colombo N., Scambia G., Kim B.G., Oaknin A., Friedlander M., Lisyanskaya A., Floquet A., Leary A., Sonke G.S., et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 8.Capoluongo E., Ellison G., López-Guerrero J.A., Penault-Llorca F., Ligtenberg M.J.L., Banerjee S., Singer C., Friedman E., Markiefka B., Schirmacher P., et al. Guidance Statement on BRCA1/2 Tumor Testing in Ovarian Cancer Patients. Semin. Oncol. 2017;44:187–197. doi: 10.1053/j.seminoncol.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Wallace A.J. New challenges for BRCA testing: A view from the diagnostic laboratory. Eur. J. Hum. Genet. 2016;24:S10–S18. doi: 10.1038/ejhg.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koczkowska M., Zuk M., Gorczynski A., Ratajska M., Lewandowska M., Biernat W., Limon J., Wasag B. Detection of somatic BRCA1/2 mutations in ovarian cancer-next-generation sequencing analysis of 100 cases. Cancer Med. 2016;5:1640–1646. doi: 10.1002/cam4.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mafficini A., Simbolo M., Parisi A., Rusev B., Luchini C., Cataldo I., Piazzola E., Sperandio N., Turri G., Franchi M., et al. BRCA somatic and germline mutation detection in paraffin embedded ovarian cancers by next-generation sequencing. Oncotarget. 2016;7:1076–1083. doi: 10.18632/oncotarget.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jonge M.M., Ruano D., van Eijk R., van der Stoep N., Nielsen M., Wijnen J.T., Ter Haar N.T., Baalbergen A., Bos M.E.M.M., Kagie M.J., et al. Validation and Implementation of BRCA1/2 Variant Screening in Ovarian Tumor Tissue. J. Mol. Diagn. 2018;20:600–611. doi: 10.1016/j.jmoldx.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Guidelines for BRCA Test Implementation: “Raccomandazioni per L’implementazione Del Test BRCA Nelle Pazienti Con Carcinoma Ovarico E Nei Familiari a Rischio Elevato Di Neoplasia”. A Cura del Gruppo di Lavoro AIOM-SIGU-SIBIOC-SIAPEC-IAP, 31 Ottobre 2018. [(accessed on 11 February 2019)]; Available online: https://www.aiom.it/pubblicazioni/raccomandazioni-position-paper/raccomandazioni-per-limplementazione-del-test-brca-nelle-pazienti-con-carcinoma-ovarico-e-nei-familiari-a-rischio-elevato-di-neoplasia/
- 14.Rigakos G., Razis E. BRCAness: Finding the Achilles heel in ovarian cancer. Oncologist. 2012;17:956–962. doi: 10.1634/theoncologist.2012-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AIFA Registry of Lynparza 2016. [(accessed on 11 February 2019)]; Available online: http://www.aifa.gov.it/content/pubblicazione-schede-di-monitoraggio-registro-lynparza-27042016.
- 16.Wong S.Q., Li J., Tan A.Y., Vedururu R., Pang J.M., Do H., Ellul J., Doig K., Bell A., MacArthur G.A., et al. Sequence artefacts in a prospective series of Formalin-Fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genom. 2014;7:23. doi: 10.1186/1755-8794-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin S., Kim Y., Chul Oh S., Yu N., Lee S.-T., Choi J.R., Lee K.-A. Validation and optimization of the Ion Torrent S5 XL sequencer and Oncomine workflow for BRCA1 and BRCA2 genetic testing. Oncotarget. 2017;23:34858–34866. doi: 10.18632/oncotarget.16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H., Thornton A., Norquist B.M., Casadei S., Nord A.S., et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennessy B.T., Timms K.M., Carey M.S., Gutin A., Meyer L.A., Flake D.D., 2nd, Abkevich V., Potter J., Pruss D., Glenn P., et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J. Clin. Oncol. 2010;28:3570–3576. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham J.M., Cicek M.S., Larson N.B., Davila J., Wang C., Larson M.C., Song H., Dicks E.M., Harrington P., Wick M., et al. Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci. Rep. 2014;4:4026. doi: 10.1038/srep04026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moschetta M., George A., Kaye S.B., Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann. Oncol. 2016;27:1449–1455. doi: 10.1093/annonc/mdw142. [DOI] [PubMed] [Google Scholar]
- 23.Kwon J. Scientific Plenary session: Costs and benefits of tumor testing for BRCA mutations in high-grade serous ovarian cancer as a triage for confirmatory genetic testing; Proceedings of the SGO 50th Annual Meeting on Womens Cancers; Honolulu, HI, USA. 16–19 March 2019. [Google Scholar]
- 24.Arts-de Jong M., de Bock G.H., van Asperen C.J., Mourits M.J., de Hullu J.A., Kets C.M. Germline BRCA1/2 mutation testing is indicated in every patient with epithelial ovarian cancer: A systematic review. Eur. J. Cancer. 2016;61:137–145. doi: 10.1016/j.ejca.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Greaves M., Maley C.C. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alizadeh A.A., Aranda V., Bardelli A., Blanpain C., Bock C., Borowski C., Caldas C., Califano A., Doherty M., Elsner M., et al. Toward understanding and exploiting tumor heterogeneity. Nat. Med. 2015;21:846–853. doi: 10.1038/nm.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patch A.-M., The Australian Ovarian Cancer Study Group. Christie E.L., Etemadmoghadam D., Garsed D.W., George J., Fereday S., Nones K., Cowin P., Alsop K., et al. Whole-Genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 28.Norquist B., Wurz K.A., Pennil C.C., Garcia R., Gross J., Sakai W., Karlan B.Y., Taniguchi T., Swisher E.M. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 2011;29:3008–3015. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swisher E.M., Sakai W., Karlan B.Y., Wurz K., Urban N., Taniguchi T. Secondary BRCA1 mutations in BRCA1-Mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loman N.J., Misra R.V., Dallman T.J., Constantinidou C., E Gharbia S., Wain J., Pallen M.J. Performance comparison of benchtop high-throughput sequencing platforms. Nat. Biotechnol. 2012;30:434–439. doi: 10.1038/nbt.2198. [DOI] [PubMed] [Google Scholar]
- 31.Bragg L.M., Stone G., Butler M.K., Hugenholtz P., Tyson G.W. Shining a light on dark sequencing: Characterising errors in Ion Torrent PGM data. PLoS Comput. Biol. 2013;9:e1003031. doi: 10.1371/journal.pcbi.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo Z.X., Wong J.C., Rozen S.G., Lee A.S.G. Evaluation and optimization of indel detection workflows for ion torrent sequecing of the BRCA1 and BRCA2 gene. BMC Genom. 2014;15:516. doi: 10.1186/1471-2164-15-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Joint Committee on Cancer (AJCC) AJCC Cancer Staging Manual. 8th ed. Springer; New York, NY, USA: 2017. [Google Scholar]
- 34.Kurman R.J., Carcangiu M.L., Herrington C.S., Young R.H. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. IARC Press; Lyon, France: 2014. [Google Scholar]
- 35.Fumagalli C., Catania C., Ranghiero A., Bosi C., Viale G., de Marinis F., Barberis M., Guerini-Rocco E. Molecular Profile of Advanced Non-Small Cell Lung Cancers in Octogenarians: The Door to Precision Medicine in Elderly Patients. J. Clin. Med. 2019;8:112. doi: 10.3390/jcm8010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fumagalli C., Vacirca D., Rappa A., Passaro A., Guarize J., Raviele P.R., De Marinis F., Spaggiari L., Casadio C., Viale G., et al. The long tail of molecular alterations in non-small cell lung cancer: A single-institution experience of next-generation sequencing in clinical molecular diagnostics. J. Clin. Pathol. 2018;71:767–773. doi: 10.1136/jclinpath-2018-205032. [DOI] [PubMed] [Google Scholar]
- 37.ENIGMA BRCA1/2 Gene Variant Classification Criteria. [(accessed on 11 February 2019)]; Available online: https://enigmaconsortium.org/wp-content/uploads/2018/10/ENIGMA_Rules_2017-06-29-v2.5.1.pdf.
- 38.BRCA Exchange Database. [(accessed on 11 February 2019)]; Available online: https://brcaexchange.org/
- 39.ClinVar Database. [(accessed on 11 February 2019)]; Available online: https://www.ncbi.nlm.nih.gov/clinvar/
- 40.Leiden Open Source Variation Database. [(accessed on 11 February 2019)]; Available online: https://databases.lovd.nl/shared/variants/BRCA1/unique.