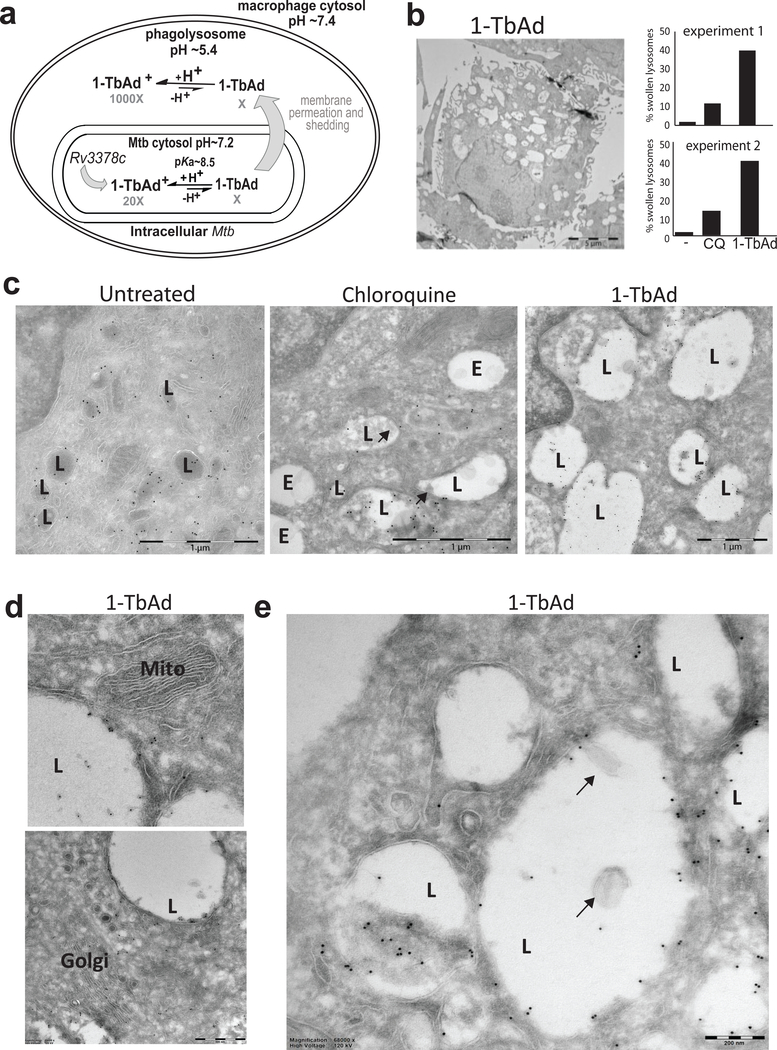
Figure 4. 1-TbAd is a lysosomotropic antacid.
a) Experiments test a compartmentalization model in which Rv3378c generates intrabacterial 1-TbAd, and the uncharged conjugate base permeates membranes. Low pH in phagolysosomes drives protonation, which incrementally raises pH and generates a charged species, 1-TbAd+, which cannot cross membranes.. b) Human macrophages generated with M-CSF and GM-CSF were treated for 2 hours with 1-TbAd (20 μM) or chloroquine (CQ) (20 μM) stained with anti-CD63 immunogold and assessed by transmission electron microscopy. An individual macrophage was counted as having swollen lysosomes when > 30 percent of cytosol was involved with CD63+ electron-lucent structures, as depicted in this low magnification image of a 1-TbAd treated cell. Images of > 150 cells were counted and subjected to the Cochran-Mantel-Haenszel test and adjusted by the method of Benjamini and Hochberg (p=5.4×10−10 for control versus 1-TbAd and p=3.2×10−5 for chloroquine versus 1-TbAd). Actual sample sizes were n=54 or 48 for controls, n=45 or 48 1-TbAd-treated, and n=70 or 46 chloroquine-treated in trial one or two, respectively. c) High magnification images (bar, 1 μm) depict lysosomes (L), as defined by anti-CD63 staining at the limiting membrane, and endosomes (E), which are electron-lucent structures lacking anti-CD63 staining. Results are representative of three biologically independent experiments with similar outcomes. d) Mitochondria (Mito) and Golgi stacks (Golgi) are recognized by membrane arrangements, which unlike lysosomes, did not show swelling or increased electron lucency. e) Higher magnification (bar, 200 nm) images show inclusions in lysosomes that contain membranes (arrows) or non-membrane bound inclusions.