Figure 4.
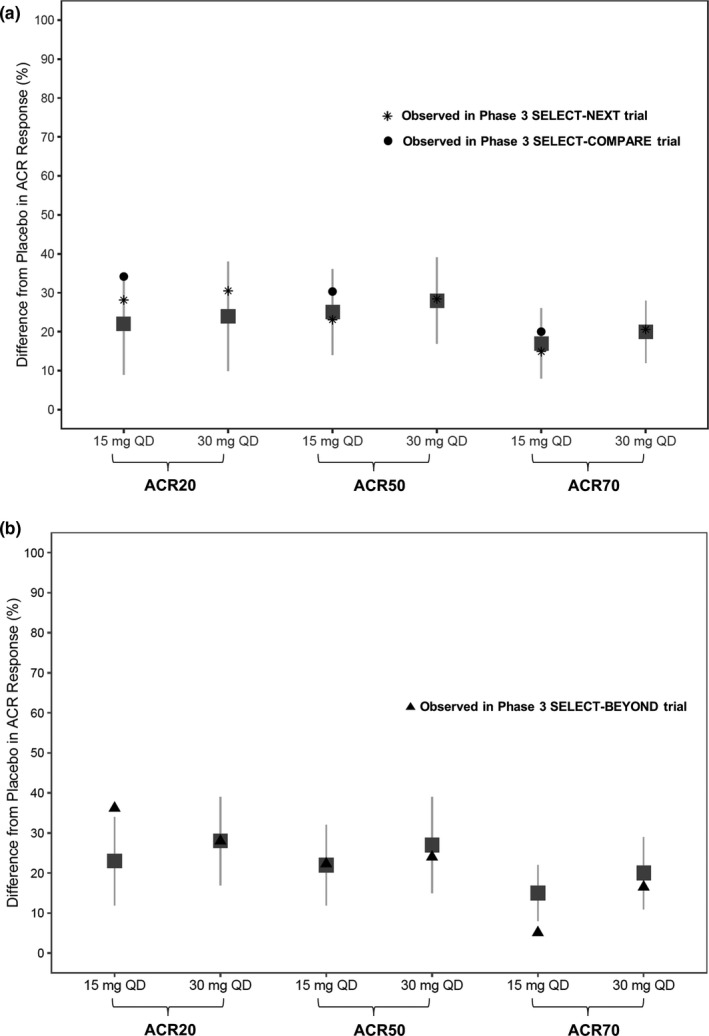
Simulated and observed American College of Rheumatology (ACR) responses (nonresponder imputation (NRI)) at week 12 in (a) methotrexate (MTX)‐inadequate responders (IRs)/conventional synthetic disease‐modifying antirheumatic drugs (csDMARD)‐IR and (b) anti‐TNF‐IR/biologics‐IR patients in phase III trials. Gray symbols and error bars represent the simulated median and 90% prediction intervals for 15 mg and 30 mg q.d. extended‐release regimens based on exposure–response analyses of the phase II studies BALANCE I and II. Black symbols represent the observed responses in phase III trials. q.d., once daily.
