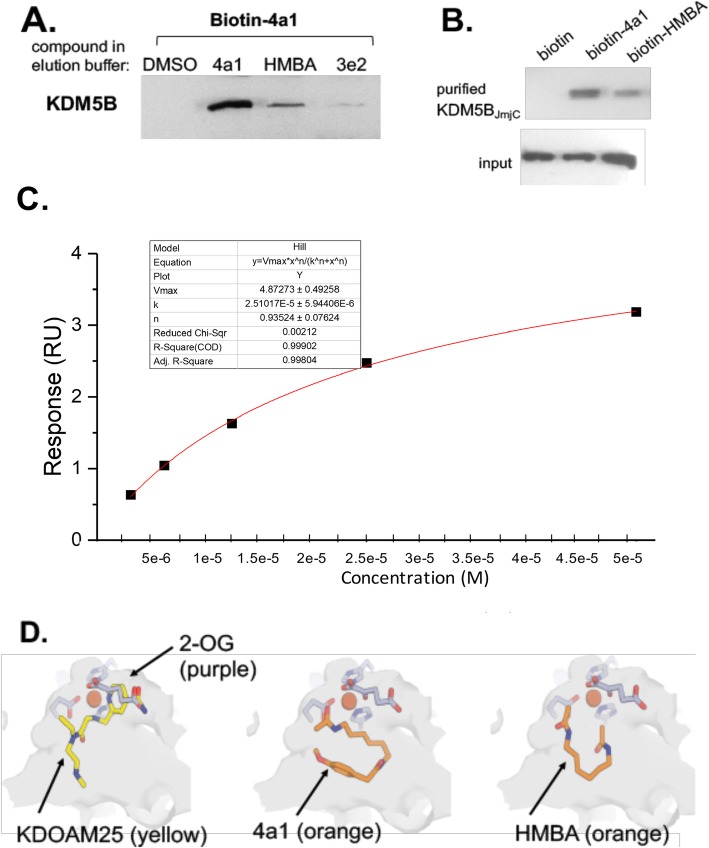
Fig. 2.
4a1 binds catalytic JmjC domain of KDM5B. a Biotin–streptavidin pull-down assay using NeutrAvidin beads bound to biotinylated 4a1 and whole cell lysates. Bound proteins were washed with buffer with DMSO or eluted using 4a1 (200 μM), HMBA (1 mM), or 3e2 (an inactive HMBA analog, 1 mM). Samples were subjected to SDS-PAGE and immunoblotted for KDM5B. b Biotin–streptavidin pull-down assay using NeutrAvidin beads bound to biotinylated 4a1 (100 μM) or biotinylated HMBA (5 mM) and purified KDM5B JmjC domain. After washes, bound proteins were eluted with SDS sample buffer and subjected to SDS-PAGE and immunoblotted for KDM5B. c Surface plasmon resonance analyses of the affinity of 4a1 for purified KDM5B JmjC domain. Figures are representative of at least three experiments. d Docking of KDOAM25, 4a1, and HMBA onto KDM5B JmjC domain