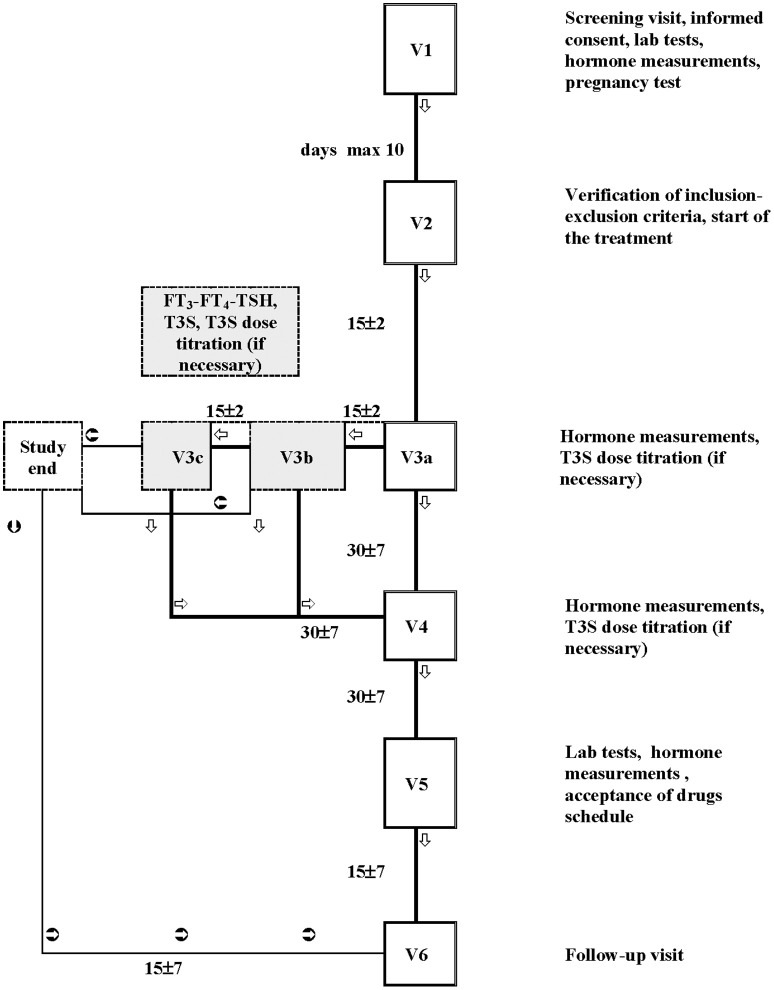
Figure 1.
Flow chart of the study. The study plan included a screening visit (Visit 1), in which patients potentially eligible were checked for inclusion and exclusion criteria; Visit 2 was performed within 10 days from Visit 1 to confirm the compliance with the inclusion and the exclusion criteria; if confirmed, the L-T4 therapy schedule was changed to L-T4+T3S. The next visits (max 3 visits: V3a, V3b, and V3c) were performed every 15 days and were dedicated to T3S titration. If during the titration period the patients maintained (or attained) the metabolic control (i.e., hormonal parameters in the accepted range), the T4+T3S dosage remained unchanged until the next follow-up visit (Visit 4, 1 month after), when a T3S dosage change was allowed. A fifth visit (visit 5) was performed after a further month of therapy. If at the end of the titration period (visit 3c) patients did not attained the metabolic control, they were removed from the study. Intermediate visit(s) were arranged in case of adverse events or whenever judged suitable for patient safety by the investigator. A safety follow-up visit (visit 6) was arranged 15 (+ maximum 7) days after visit 5.