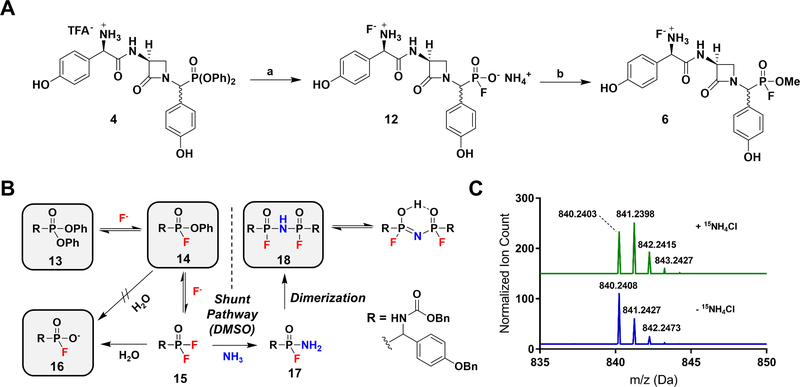
Figure 2.
(A). Synthesis of FP 6. Reaction conditions: (a) NH4F, ACN, 60 oC, quant.; (b) CH2N2, DMSO. (B). Proposed fluorinative hydrolysis mechanism. Intermediates 13, 14, 16, and 18 were directly observed by 31P-NMR and UPLC-HRMS. (C). HRMS of 18 supplemented (top) and unsupplemented (bottom) with 15NH4Cl indicating by isotopic mass shift the incorporation of 15N into the molecule.