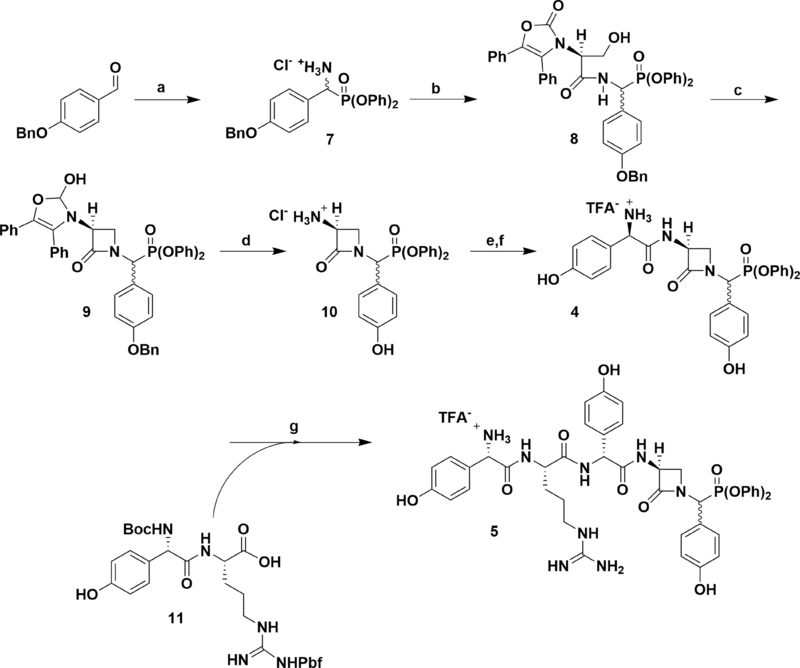
Scheme 1.
Synthesis of nocGP(OPh)2 and pro-nocGP(OPh)2 C-Terminal Epimers. Reaction conditions: (a) (1) BocNH2, P(OPh)3, AcOH, (2) TFA, DCM, (3) NaHCO3, Et2O, (4) HCl, THF, 75% over four steps; (b) l-Ox-Ser-DCA, PyBOP, DIPEA, DMF, 68%; (c) P(OEt)3, DEAD, THF, 65%; (d) cat. 10% Pd/C, 50 psi H2, HCl, EtOAc/EtOH, 62%; (e) (1) L-Boc-Hpg, isobutylchloroformate, 2,6-lutidine, cat. N-methylmorpholine, acetone, (2), 10, 2,6-lutidine, DMF, 69% over two steps; (f) TFA, DCM, quant.; (g) (1) N-Boc-L-Hpg-NG-Pbf-L-Arg (11), PyBOP, DIPEA, DMF, (2) TFA, (3) HPLC, 25% over three steps.