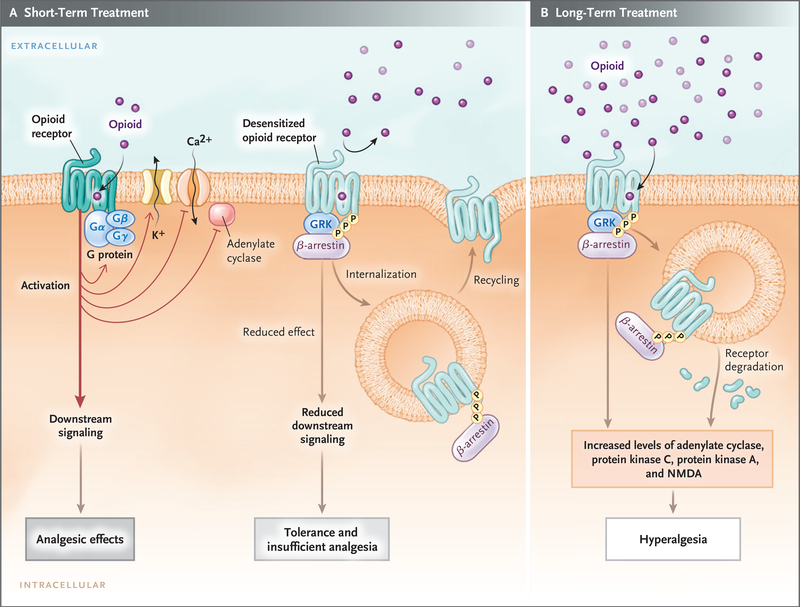
Figure 2. Opioid-Receptor Signaling during Short-Term Therapy and Long-Term Therapy.
In short-term treatment, the binding of an opioid to its receptor (Panel A) causes downstream G-protein–coupled receptors, composed of Gαβγ subunits, to dissociate into Gα and Gβγ subunits. The dissociated G-protein subunits inhibit voltage-gated calcium channels by means of reduced transmitter release, activate inward-rectifying potassium channels (causing hyperpolarization of the membrane), and inhibit downstream adenylate cyclase enzymes, decreasing cyclic adenosine monophosphate levels. These events reduce excitability and nociception and result in analgesic effects. When an opioid binds to its receptor, it becomes an immediate substrate for phosphorylation by G-protein–coupled receptor kinase (GRK), which leads to recruitment and binding of β-arrestin protein to the receptor. This results in desensitization and sometimes endocytosis of the receptor; each of these events decreases the responses to opioids, inducing tolerance and insufficient analgesia. Opioid-receptor signaling terminates when the opioid is displaced from the receptor. After the stimulus (i.e., the agonist) is withdrawn, the desensitized receptor recovers over time (minutes to hours, depending on the agonist), Gα rebinds to Gβγ and once again forms Gαβγ, and the endocytosed receptor is reexpressed on the plasma membrane in a resensitized state. In long-term treatment (Panel B), escalating doses of opioids and concomitant persistent activation of the receptor lead to aggravation of the tolerance by receptor-dependent and receptor-independent intracellular signaling changes, which include up-regulation of the antiopioid (pro-nociceptive) signaling pathways. The sustained β-arrestin binding to the receptor often leads to internalization, degradation, and down-regulation of membrane receptor number, further decreasing response to opioids. Receptor down-regulation occurs with some opioids (e.g., fentanyl) but not others (e.g., morphine). Phosphorylation by other kinases (e.g., protein kinases A and C), increased adenylate cyclase activity (with increased cyclic adenosine monophosphate levels), activation of N-methyl-d-aspartate (NMDA) receptor, and down-regulation of glutamate receptors (increased glutamate levels) are all implicated in the imbalance between pro-nociceptive and antinociceptive pathways, which results in attenuated analgesic effects, aggravated pain behaviors, increased tolerance, and opioid-induced hyperalgesia.