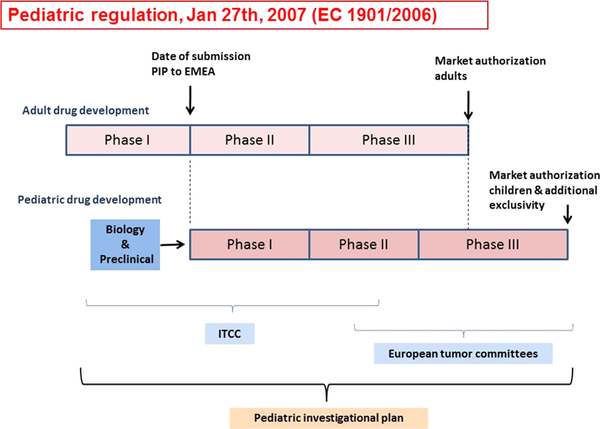
Fig 16.
A schematic representation of paediatric drug development. The new European Committee legislation (Regulation EC No. 1901/2006; the ‘Paediatric Regulation’) stimulates pharmaceutical companies to consider paediatric indications when they want to authorise a new medicinal product. Plans for the paediatric development of a drug need to be submitted to the European Medicines Agency (EMEA), in a so-called ‘Paediatric Investigation Plan or PIP’, which comprises the entire development process from pre-clinical studies to clinical development. While the PIP should be submitted following the phase I development in adults, the time to start the paediatric development is defined on a case by case approach, as additional safety and efficacy data may be required before launching the first paediatric studies. A PIP is legally binding but can be amended when the development process requires changes. When pharmaceutical companies have performed paediatric studies they will be rewarded by an additional period of market exclusivity. The Innovative Therapies for Children with Cancer consortium has the facilities for both pre-clinical as well as early clinical development, which is performed in close collaboration with the European tumour committees, who are typically responsible for standard of care and late phase II/phase III studies. Reprinted from Cancer Treatment Reviews, 36, Zwaan, C.M., Kearns, P., Caron, H., Verschuur, A., Riccardi, R., Boos, J., Doz, F., Geoerger, B., Morland, B., Vassal, G., The role of the ‘innovative therapies for children with cancer’ (ITCC) European consortium., 328–334. Copyright 2010, with permission from Elsevier.