Abstract
The development of robust oxygen-tolerant reversible deactivation radical polymerization (RDRP) systems can dramatically simplify synthesis of well-defined polymers without redundant deoxygenation procedures. Herein, we broaden the application of oxygen-tolerant RDRP from homogeneous aqueous and organic solutions to dispersed media. The glucose oxidase (GOx) degassed atom transfer radical polymerization (ATRP) was conducted in miniemulsion and ab initio emulsion systems using various catalyst regeneration methods: ARGET, ICAR, photo, and eATRP. All of these polymerization procedures led to polymers with predetermined molecular weight, low dispersity, and high chain-end functionality. Emulsion ATRP on a larger scale with preserved control was also successful. Circular dichroism (CD) studies demonstrated that the anionic surfactant, sodium dodecyl sulfate (SDS), did not damage the secondary structure of GOx. This confirms the versatility of the GOx system for various low ppm ATRP methods. GOx-assisted oxygen removal in the synthesis of hydrophobic polymers is reported for the first time. The low cost of deoxygenation reagents and the ability to scale up the procedure suggest the potential for industrial production, especially for emulsion polymerization.
Graphical Abstract
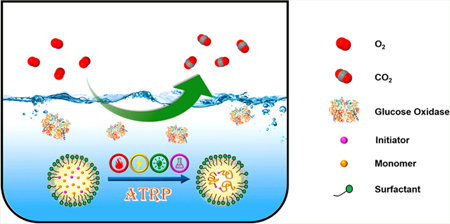
Reversible deactivation radical polymerization (RDRP) techniques have been widely studied and utilized to synthesize polymers with predetermined molecular weight and low dispersity. RDRP methods are compatible with a broad range of functional groups and solvents and permit the synthesis of polymers with well-defined molecular architecture.1 Atom transfer radical polymerization (ATRP)2–5 and reversible addition–fragmentation (RAFT) polymerization6,7 are among the most often used RDRP techniques, where the fraction of terminated chains is suppressed and chains grow concurrently.
Miniemulsion and emulsion procedures, used in dispersed aqueous media, are characterized by efficient heat transfer, low viscosity, and lack of volatile organic solvents.8 Thus, polymerization in dispersed media is increasingly favored in both laboratory and industry. Indeed, many polymer emulsions are commercial products. RDRP in dispersed media can be more efficient than in homogeneous media for the synthesis of star, brush, and multiblock polymers, as the compartmentalization effect further suppresses radical termination.9
Oxygen inhibits radical polymerizations.10 O2 reacts with C-based radicals to form low reactivity peroxy radicals. Also, it oxidizes the ATRP activator, typically a CuIL species to form the CuIIL deactivator species, thus gradually quenching the polymerization. Commonly used laboratory degassing techniques include freeze–pump–thaw cycles, purging with inert gas, and working in a glovebox or Schlenk line. These methods are time-consuming, difficult to scale up, and challenging for nonexperts and may lead to significant evaporation of some volatile reagents. It would therefore be advantageous to design a chemical degassing procedure that is orthogonal to RDRP.
In recent years, four types of oxygen-tolerant RDRP systems have been developed.10 One is polymerizing “through oxygen”, wherein a limited amount of O2 is consumed by the propagating radicals and peroxides are incorporated in the polymer chain. This happens with a relatively high flux of radicals11 and with a small headspace in the reaction vessel.12 Another method used for both ATRP and RAFT is enzyme degassing, where the oxidation of glucose catalyzed by GOx consumes oxygen rapidly and generates H2O2. The high activity and high stability of GOx make this system attractive for radical polymerization.13–18 However, in ATRP, H2O2 could interact with Cu(I) species via Fenton-like reaction and generate additional radicals, leading to new chains. This problem could be addressed by addition of sodium pyruvate to transform H2O2 to inert species.18 A third method is based on activator regeneration (ARGET ATRP), where reducing agents, such as ascorbic acid (AsAc) or tin(II), scavenge oxygen.12,19,20 Lastly, photochemical RAFT systems, including photoinduced electron/energy transfer (PET)-RAFT, photo-induced RAFT, and photoiniferter RAFT polymerizations, also exhibit oxygen tolerance.21–24 In PET-RAFT, triplet oxygen is rapidly transformed to singlet oxygen in the presence of singlet oxygen quenchers.21,22,25,26
Most of the methods mentioned above have been reported in both organic and aqueous media. However, the enzyme-degassing system in the presence of GOx has so far required aqueous media (or water/highly polar solvent mixture) to retain activity,27,28 and this method has only been used for preparation of water-soluble polymers. The oxygen-tolerant RDRP in dispersed media was only once reported. The activators generated by electron transfer (AGET) ATRP were conducted by the addition of excess ascorbic acid to consume the oxygen.20 However, evaluating the appropriate dosage of AsAc for the polymerization is challenging. The required amount depends not only on polymerization kinetics but also on the flask size, reaction volume, and head space.
In this paper, we report the application of GOx-deoxygenated ATRP in miniemulsion and emulsion. The polymerizations were controlled by a Cu/TPMA/DS– (tris(2-pyridylmethyl)amine)/dodecyl sulfate) ion-pair catalyst.29–32 The reaction conditions were adapted from nitrogen-degassed systems. This enzyme-assisted deoxygenation was applied to various ATRP techniques, and it was tested for polymerization of acrylates and methacrylates. The scalability was also investigated.
Table 1 shows the composition of miniemulsion and emulsion ARGET ATRP using the GOx-degassing method in the presence of glucose and pyruvate.18 The procedure used for oxygen-tolerant miniemulsion ATRP utilizing an ion-pair catalyst was straightforward. Glucose and sodium pyruvate were dissolved in the aqueous solution of NaBr and SDS. The aqueous solution was mixed with the organic solution and homogenized by ultrasonication to form a white miniemulsion. Finally, GOx was added to the miniemulsion, and the flask was capped with a rubber stopper and stirred for 3 min. Polymerization was then triggered by feeding AsAc aqueous solution to the reaction (Scheme 1).
Table 1.
Composition of Organic and Aqueous Phases in Miniemulsion and Emulsion ARGET ATRP of BMA
| componenta | weight (g) | comments |
|---|---|---|
| Organic Phase | ||
| BMA | 1.79 | 20 vol % (18 wt %) to total |
| EBPA | 0.015 | initiator in miniemulsion ATRP, [BMA]/[EBPA] = 280/1 |
| hexadecane | 0.19 | 10.8 wt % to BMA (used ONLY for |
| (HD) | miniemulsion) | |
| Aqueous | ||
| Phase | ||
| water | 8 | distilled water |
| PEO2k-BPA | 0.053 | initiator in emulsion ATRP, [BMA]/[PEO2k-BPA] = 500/1 |
| SDS | 0.082 | 4.6 wt % to BMA |
| NaBr | 0.10 | [NaBr] = 0.1 M |
| CuIIBr2 | 2.2 × 10−3 | 1 mM with respect to Vtotal |
| TPMA | 2.9 × 10−3 | [CuIIBr2J/[TPMA] = 1/1 |
| glucose | 0.36 | [glucose] = 0.2 M |
| sodium | 0.11 | [sodium pyruvate] = 0.1 M |
| pyruvate | ||
| GOx | 3.2 × −3 | 2 μM, added 3 min before reaction |
| AsAc | fed at 6.5 × 10−4 g/h |
Conditions: T = 45 °C. Vtotal = 10 mL. The miniemulsion was prepared by ultrasonication, and the emulsion formed during polymerization. See details in the Supporting Information.
Scheme 1.
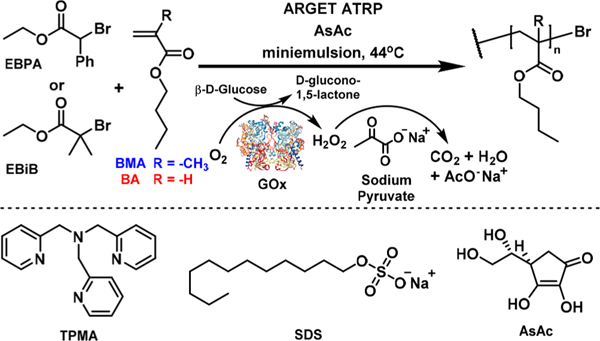
Miniemulsion ARGET ATRP of BMA and BA Using GOx Deoxygenating System
Miniemulsion ARGET ATRPs of n-butyl methacrylate (BMA) and n-butyl acrylate (BA) were carried out using the GOx deoxygenating system (Figure 1). The molar ratio was [M]/[I]/[CuBr2]/[TPMA] = 280/1/0.22/0.22 (I = ethyl α-bromophenylacetate (EBPA) for BMA and ethyl α-bromoiso- butyrate (EBiB) for BA). The feeding rate of AsAc was 3.7 μmol/h. Well-controlled polymers were synthesized in a few hours. For BMA, 89% conversion was reached after 6.5 h, yielding polymers with Mn,exp = 32 400 (vs Mn,th = 35 600) and low dispersity, 1.16. The polymerization of BA reached 71% conversion after 20 h. The Mn = 25 300 of the final polymer was close to its theoretical value (25 700) with a low dispersity, 1.24. The zeta-average PBA particle size was 114.6 ± 0.8 nm, which was not influenced by addition of GOx, glucose, and sodium pyruvate.30 The chain-end functionality of PBA was demonstrated by successful chain extension by tert-butyl acrylate (tBA) (Figure S1). Thus, enzyme-deoxygenated miniemulsion ARGET ATRP was successfully applied to polymerization of both acrylates and methacrylates.
Figure 1.
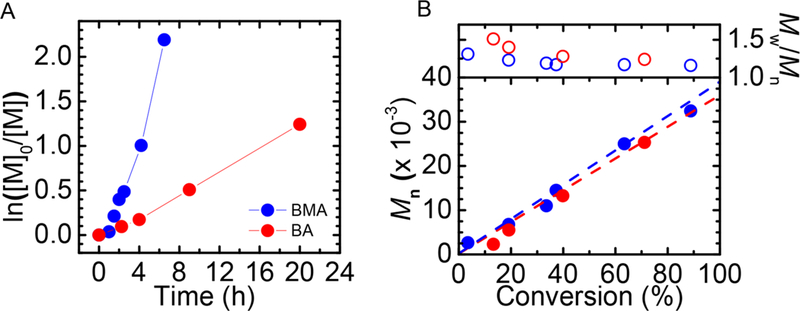
GOx-degassed miniemulsion ARGET ATRP of BMA and BA. (A) Kinetics and (B) evolution of Mn and Đ. Reaction conditions: BMA or BA (monomer, M) 2 mL (20 vol %), [M]/[I]/[CuBr2/TPMA] = 280/1/0.2, [HD] = 10 wt % relative to M, [SDS] = 4.6 wt % relative to M, [NaBr] = 0.1 M, [glucose] = 0.2 M, [GOx] = 2 μM, [sodium pyruvate] = 0.1 M, T = 44 °C. AsAc was fed at a rate of 3.7 μmol/h.
In addition to the enzyme-assisted degassing, traditional nitrogen bubbling and polymerization without any deoxygenation were also conducted (Figures S2–S3). Nitrogen purging is an efficient method to remove oxygen prior to polymerization and is commonly used in RDRP. The rate of polymerization using GOx was higher than with nitrogen purging due to a more thorough deoxygenation. As continuous injection of AsAc could facilitate consumption of oxygen, a control experiment without any degassing was carried out. After more than 6 h induction period, 10% conversion was observed after the next 12 h, but the molecular weight was twice as high as the theoretical value, and the dispersity was high (1.44), indicating poor initiation efficiency and inefficient control. Thus the GOx-degassing system increased the rate of polymerization and provided superior control compared to other methods.
The ion-pair catalysis involving the Cu/TPMA/DS– was applied to electrochemically mediated ATRP (eATRP) and photoinduced ATRP (photo-ATRP), respectively (Figure 2). Using the same conditions as in the original reports,29,32 well-controlled polymerizations were successfully carried out without nitrogen purging by simple addition of glucose, GOx, and sodium pyruvate. All miniemulsion polymerizations were triggered without a significant induction period and led to polymers with low dispersity.
Figure 2.
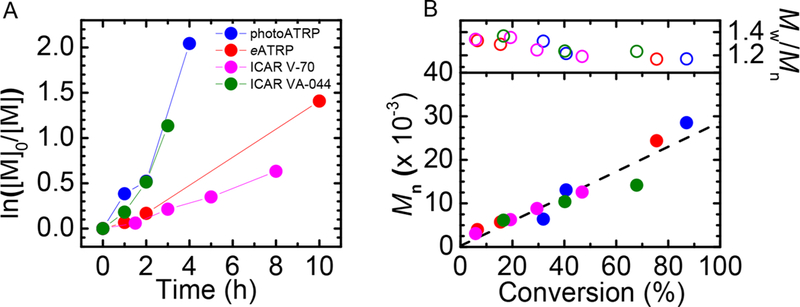
GOx-degassed miniemulsion photoATRP, eATRP and ICAR ATRP of BMA. (A) Kinetics and (B) evolution of Mn and Đ. Reaction conditions: BMA 2 mL (20 vol %), [BMA]/[EBPA]/[CuBr2] = 200/1/0.16, [HD] = 10 wt % relative to BMA, [SDS] = 4.6 wt % relative to BMA, [NaBr] = 0.1 M, [glucose] = 0.2 M, [GOx] = 2 μM, [sodium pyruvate] = 0.1 M, T = 44 °C (except ICAR ATRP using V-70 at 30 °C). PhotoATRP--[CuBr2]/[TPMA] = 1/3, λmax = 365 nm, intensity = 5 mW/cm2; eATRP–[CuBr2]/[TPMA] = 1/1, Eapp = Epc, CuTPMA, working electrode = Pt mesh; ICAR ATRP--[CuBr2]/[TPMA] = 1/1, TI/EBPA = 0.3/1, TI = VA-044 or V-70.
ICAR ATRP with thermal radical initiators (TIs) with different water solubility was then investigated. According to previous reports,29 over 95% of Cu resides on the surface of the oil droplets, thus both hydrophilic and hydrophobic radicals could access the Cu catalysts and trigger polymerization. The water-soluble VA-044 and oil-soluble V-70 had t½ = 10 h at 44 and 30 °C, respectively. To ensure the similar rate of initiator dissociation, the ratio of [EBPA]/[TI] was fixed at 1/0.3, and the two ICAR ATRP reactions were carried out at these temperatures. The polymerization induced by V-70 was slower because the rate of activation (kact),33 ATRP equilibrium constant (KATRP), and rate of propagation (kp) of BMA all increase with temperature.34 Yet in all aforementioned ATRP systems, the obtained polymers had predetermined molecular weights and low dispersities (Figure 2).
The GOx deoxygenated system was then applied to a standard emulsion. Conducting emulsion polymerization does not require high sheer forces and thus is more energy efficient. The key to synthesize polymers with predetermined molecular weight is to avoid pre-emulsification and maintain a low stirring rate.31 Previously, the aqueous solution, monomer, and AsAc solution had to be degassed separately. Employing the GOx deoxygenation system avoided this problem. The aqueous solution of initiator PEG2k-BPA, SDS, NaBr, glucose, and sodium pyruvate was mixed with GOx, and then BMA was slowly injected to the flask without stirring. AsAc was fed into the flask at a stirring rate of 250 rpm. The monomer slowly diffused to the formed polymer micelles, and the organic layer disappeared after one hour. The conversion reached 60% after 4 h, and the molecular weight matched the theoretical value with dispersity as low as 1.15 (Figure 3). The final zeta-average particle size was 184.6 ± 1.8 nm.
Figure 3.

GOx degassed ab initio emulsion ARGET ATRP of BMA using different total volumes. (A) Kinetics and (B) evolution of Mn and Đ. Conditions: [BMA]/[PEO2kBPA]/[NaBr]/[CuBr2/TPMA] = 500/1/20/0.2, BMA 20% (v/v) in H2O, SDS was 18.4 wt % to BMA. [glucose] = 0.2 M, [GOx] = 2 μM, [sodium pyruvate] = 0.1 M. Vtotal = 10 or 80 mL. T = 44 °C (10 mL) 0.8; (80 mL) 6.4 μmol of AsAc injected at t = 0, then AsAc was fed with a syringe pump, feeding rate = (10 mL) 0.8; (80 mL) 6.4 μmol/h. Stirring rate = 250 rpm.
Since emulsion polymerization is widely used in industrial applications, the feasibility of scaling up the GOx-deoxy-genated emulsion ATRP was tested. The total volume was increased to 80 mL using identical molar ratios of reagents (Figure 3). The rate of polymerization slightly decreased due to less efficient stirring in the larger volume flask. Nevertheless, over 60% conversion was reached in 8 h, and the molecular weight of the final polymer agreed perfectly with the theoretical value and had low dispersity. Since glucose, GOx, and sodium pyruvate are very inexpensive, this system can be attractive for industry.
SDS is a commonly used surfactant, but it is also often used to denature proteins by disrupting noncovalent peptide bonds. Thus, SDS could destroy enzymes. Therefore, circular dichroism (CD) measurements were carried out to examine the viability of the enzyme. In all of the ATRP experiments, 4.6 wt % SDS vs monomer was used, i.e., 28.5 mM. The same concentration of SDS was used with GOx in deionized water for 2 h and compared to a control group, without added SDS, and another experiment in which SDS was added just before the measurement.
In the CD spectra (Figure 4), signals of all samples perfectly overlapped, indicating the unchanged secondary structure of GOx. This is in agreement with a previous report,35 where SDS did not show any rapid effects on the stability of GOx.
Figure 4.
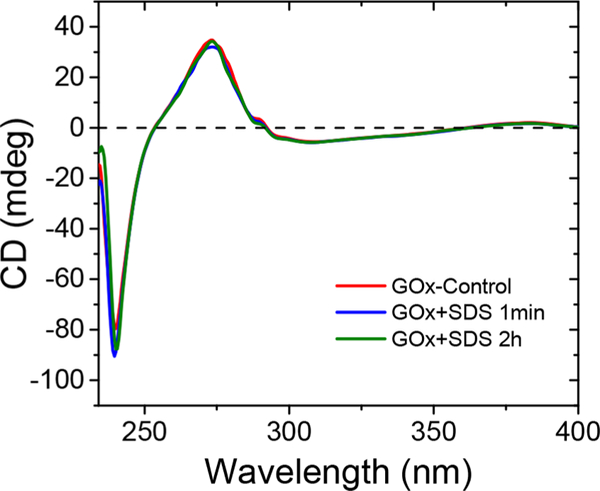
CD spectra of GOx solution in the absence and presence of SDS. [GOx] = 2 μM, [SDS] = 28.5 mM, T = 44 °C.
In conclusion, oxygen-tolerant ATRP using GOx as oxygen scavenger was conducted in miniemulsion and emulsion ATRP using various low ppm ATRP techniques. The GOx deoxygenation process is greener than nitrogen purging, as monomer evaporation is suppressed. The compatibility of the GOx deoxygenation system with several ATRP methods and monomers was confirmed. The stability of GOx in miniemulsion was demonstrated by both polymerization results and CD analysis. The application of GOx was expanded to dispersed media and constitutes the first report of GOx-assisted synthesis of hydrophobic polymers with some industrial potential.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. James Spanswick and Emilee Tkacik for useful suggestions. The financial support from NSF CHE 170749 and NIH (R01DE020843) is acknowledged.
Footnotes
■ ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmacro-lett.8b00711.
Detailed experimental procedure, polymerization kinetics, and molecular weights (PDF)
The authors declare no competing financial interest.
■REFERENCES
- (1).Braunecker WA; Matyjaszewski K Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32 (1), 93–146. [Google Scholar]
- (2).Matyjaszewski K; Xia JH Atom transfer radical polymerization. Chem. Rev. 2001, 101 (9), 2921–2990. [DOI] [PubMed] [Google Scholar]
- (3).Matyjaszewski K Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45 (10), 4015–4039. [Google Scholar]
- (4).Matyjaszewski K; Tsarevsky NV Macromolecular Engineering by Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2014, 136 (18), 6513–6533. [DOI] [PubMed] [Google Scholar]
- (5).Matyjaszewski K Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441. [DOI] [PubMed] [Google Scholar]
- (6).Chiefari J; Chong YK; Ercole F; Krstina J; Jeffery J; Le TPT; Mayadunne RTA; Meijs GF; Moad CL; Moad G; Rizzardo E; Thang SH Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31 (16), 5559–5562. [Google Scholar]
- (7).Moad G; Chiefari J; Chong YK; Krstina J; Mayadunne RTA; Postma A; Rizzardo E; Thang SH Living free radical polymerization with reversible addition-fragmentation chain transfer (the life of RAFT). Polym. Int. 2000, 49 (9), 993–1001. [Google Scholar]
- (8).Asua JM Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27 (7), 1283–1346. [Google Scholar]
- (9).Zetterlund PB; Kagawa Y; Okubo M Controlled/living radical polymerization in dispersed systems. Chem. Rev. 2008, 108 (9), 3747–3794. [DOI] [PubMed] [Google Scholar]
- (10).Yeow J; Chapman R; Gormley AJ; Boyer C Up in the air: oxygen tolerance in controlled/living radical polymerisation. Chem. Soc. Rev. 2018, 47 (12), 4357–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Calitz FM; Tonge MP; Sanderson RD Kinetic and electron spin resonance analysis of RAFT polymerization of styrene. Macromolecules 2003, 36 (1), 5–8. [Google Scholar]
- (12).Liarou E; Whitfield R; Anastasaki A; Engelis NG; Jones GR; Velonia K; Haddleton DM Copper-Mediated Polymerization without External Deoxygenation or Oxygen Scavengers. Angew. Chem., Int. Ed. 2018, 57 (29), 8998–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).O’malley JJ; Ulmer RW Thermal stability of glucose oxidase and its admixtures with synthetic polymers. Biotechnol Bioeng. 1973, 15, 917–925. [DOI] [PubMed] [Google Scholar]
- (14).Iwata H; Hata Y; Matsuda T; Ikada Y Initiation Of Radical Polymerization by Glucose-Oxidase Utilizing Dissolved-Oxygen. J. Polym. Sci., Part A: Polym. Chem. 1991, 29 (8), 1217–1218. [Google Scholar]
- (15).Oytun F; Kahveci MU; Yagci Y Sugar overcomes oxygen inhibition in photoinitiated free radical polymerization. J. Polym. Sci., Part A: Polym. Chem. 2013, 51 (8), 1685–1689. [Google Scholar]
- (16).Lv Y; Liu ZF; Zhu AQ; An ZS Glucose oxidase deoxygenation-redox initiation for RAFT polymerization in air. J. Polym. Sci., Part A: Polym. Chem. 2017, 55 (1), 164–174. [Google Scholar]
- (17).Liu ZF; Lv Y; An ZS Enzymatic Cascade Catalysis for the Synthesis of Multiblock and Ultrahigh-Molecular-Weight Polymers with Oxygen Tolerance. Angew. Chem. Int. Ed. 2017, 56 (44), 13852–13856. [DOI] [PubMed] [Google Scholar]
- (18).Enciso AE; Fu LY; Russell AJ; Matyjaszewski K A Breathing Atom-Transfer Radical Polymerization: Fully Oxygen-Tolerant Polymerization Inspired by Aerobic Respiration of Cells. Angew. Chem., Int. Ed. 2018, 57 (4), 933–936. [DOI] [PubMed] [Google Scholar]
- (19).Min K; Gao HF; Matyjaszewski K Preparation of homopolymers and block copolymers in miniemulsion by ATRP using activators generated by electron transfer (AGET). J. Am. Chem. Soc. 2005, 127 (11), 3825–3830. [DOI] [PubMed] [Google Scholar]
- (20).Min K; Jakubowski W; Matyjaszewski K AGET ATRP in the presence of air in miniemulsion and in bulk. Macromol. Rapid Commun. 2006, 27 (8), 594–598. [Google Scholar]
- (21).Xu JT; Jung K; Atme A; Shanmugam S; Boyer C A Robust and Versatile Photoinduced Living Polymerization of Conjugated and Unconjugated Monomers and Its Oxygen Tolerance. J. Am. Chem. Soc. 2014, 136 (14), 5508–5519. [DOI] [PubMed] [Google Scholar]
- (22).Xu JT; Jung K; Boyer C Oxygen Tolerance Study of Photoinduced Electron Transfer-Reversible Addition-Fragmentation Chain Transfer (PET-RAFT) Polymerization Mediated by Ru(bpy) (3) Cl-2. Macromolecules 2014, 47 (13), 4217–4229. [Google Scholar]
- (23).Corrigan N; Xu JT; Boyer C A Photoinitiation System for Conventional and Controlled Radical Polymerization at Visible and NIR Wavelengths. Macromolecules 2016, 49 (9), 3274–3285. [Google Scholar]
- (24).Fu Q; Xie K; McKenzie TG; Qiao GG Trithiocarbonates as intrinsic photoredox catalysts and RAFT agents for oxygen tolerant controlled radical polymerization. Polym. Chem. 2017, 8 (9), 1519–1526. [Google Scholar]
- (25).Shanmugam S; Xu JT; Boyer C Exploiting Metal-loporphyrins for Selective Living Radical Polymerization Tunable over Visible Wavelengths. J. Am. Chem. Soc. 2015, 137 (28), 9174–9185. [DOI] [PubMed] [Google Scholar]
- (26).Corrigan N; Rosli D; Jones JWJ; Xu JT; Boyer C Oxygen Tolerance in Living Radical Polymerization: Investigation of Mechanism and Implementation in Continuous Flow Polymerization. Macromolecules 2016, 49 (18), 6779–6789. [Google Scholar]
- (27).Chapman R; Gormley AJ; Herpoldt KL; Stevens MM Highly Controlled Open Vessel RAFT Polymerizations by Enzyme Degassing. Macromolecules 2014, 47 (24), 8541–8547. [Google Scholar]
- (28).Schneiderman DK; Ting JM; Purchel AA; Miranda R; Tirrell MV; Reineke TM; Rowan SJ Open-to-Air RAFT Polymerization in Complex Solvents: From Whisky to Fermentation Broth. ACS Macro Lett. 2018, 7 (4), 406–411. [DOI] [PubMed] [Google Scholar]
- (29).Fantin M; Chmielarz P; Wang Y; Lorandi F; Isse AA; Gennaro A; Matyjaszewski K Harnessing the Interaction between Surfactant and Hydrophilic Catalyst To Control eATRP in Miniemulsion. Macromolecules 2017, 50 (9), 3726–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Wang Y; Lorandi F; Fantin M; Chmielarz P; Isse AA; Gennaro A; Matyjaszewski K Miniemulsion ARGET ATRP via Interfacial and Ion-Pair Catalysis: From ppm to ppb of Residual Copper. Macromolecules 2017, 50 (21), 8417–8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lorandi F; Wang Y; Fantin M; Matyjaszewski K Ab Initio Emulsion Atom-Transfer Radical Polymerization. Angew. Chem. Int. Ed. 2018, 57 (27), 8270–8274. [DOI] [PubMed] [Google Scholar]
- (32).Wang Y; Dadashi-Silab S; Matyjaszewski K Photoinduced Miniemulsion Atom Transfer Radical Polymerization. ACS Macro Lett. 2018, 7 (6), 720–725. [DOI] [PubMed] [Google Scholar]
- (33).Seeliger F; Matyjaszewski K Temperature Effect on Activation Rate Constants in ATRP: New Mechanistic Insights into the Activation Process. Macromolecules 2009, 42 (16), 6050–6055. [Google Scholar]
- (34).Krys P; Matyjaszewski K Kinetics of Atom Transfer Radical Polymerization. Eur. Polym. J. 2017, 89, 482–523. [Google Scholar]
- (35).Moosavimovahedi AA; Housaindokht MR; Moghaddasi J Thermodynamic Studies Of the Interaction Of Glucose-Oxidase with Anionic And Cationic Surfactants. Thermochim. Acta 1993, 219, 143–150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


