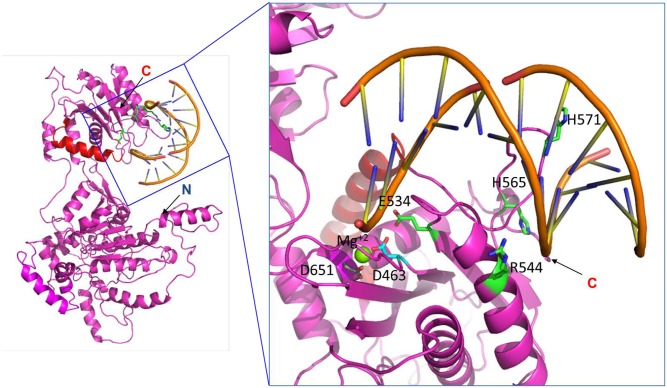
Fig 5. Predicted structure of the DNA binding motif of full-length wild-type pUL89.
The left figure shows a ribbon representation of full-length pUL89 and double stranded DNA. Residues 580–600, expected to be responsible for interaction with pUL56, are colored in red. The figure on the right represents a magnified view of the local structure from the boxed region highlighting the predicted functional motifs and including high-resolution data for the C-terminal part of pUL89 (Nadal et al.; 25). Nadal et al. (2010) identified the nuclease domain comprised of D463, E534 and D651 and alluded to a Mg2+ dependent mechanism (Mg2+ is shown in green). In contrast to this, by using the full-length pUL89, this report confirms D463 (cyan) as an amino acid required for nuclease activity and argine 544 as critical for binding double stranded DNA (shown in green).