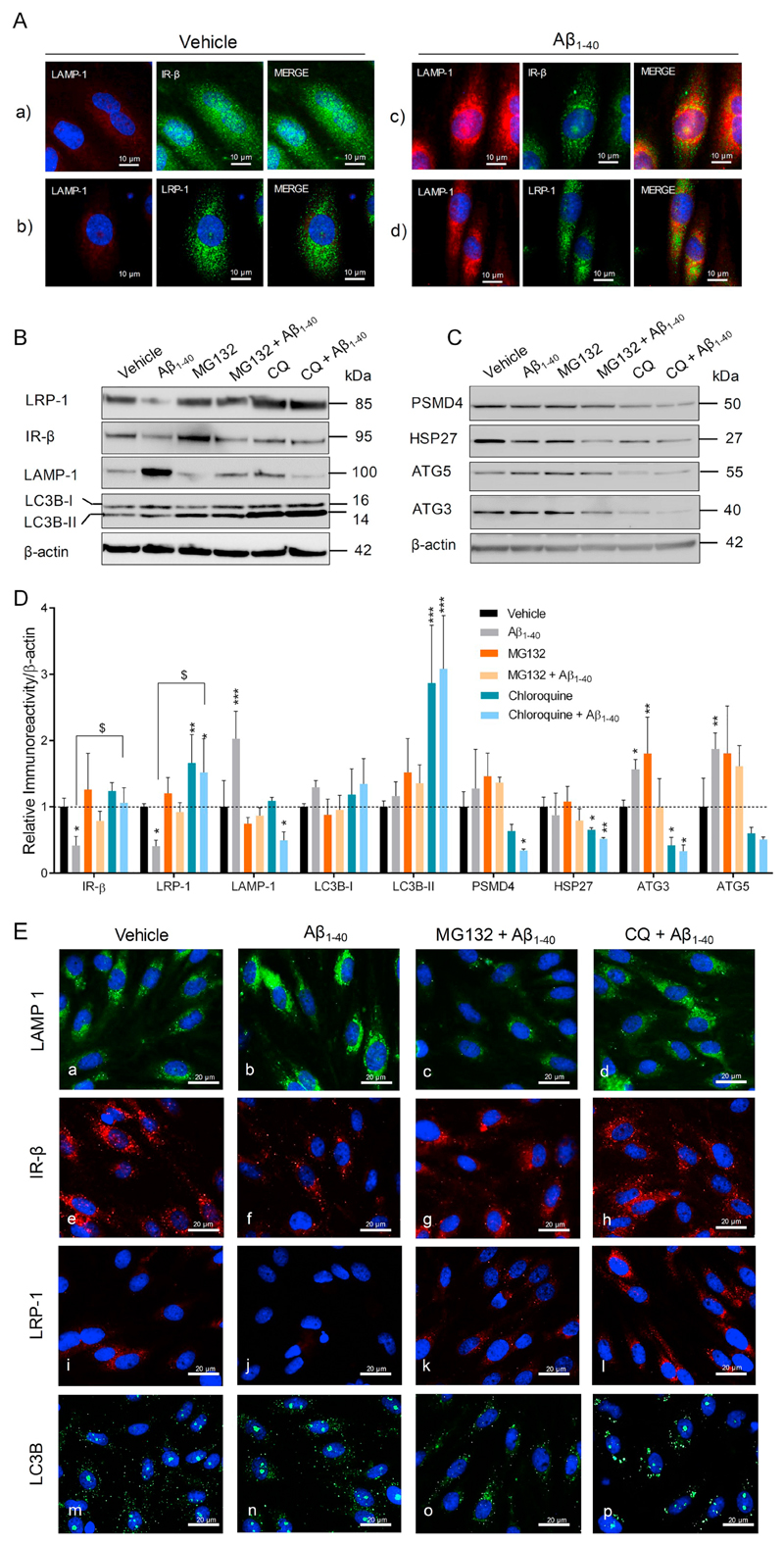
Fig. 8.
Aβ aggravates LRP-1 and IR-β degradation by inducing autophagy-lysosomal activity in pBCEC. Freshly isolated pBCEC were seeded on collagen-G coated 6 well plates and coated chamber slides. Cells were serum starved for 6 h and incubated with vehicle or freshly reconstituted Aβ1–40 (240 nM) for 6 h. (A) Double immune-fluorescent stainings of (a, c) LAMP-1 (red) and IR-β (green), (b, d) LAMP-1 (red) and LRP-1 (green). (B–D) Cells were incubated with vehicle or Aβ1–40 (240 nM), with or without chloroquine (30 μM) and MG132 (20 μM) for 6 h under serum free conditions, lysed and subjected to immune-blotting (B, C) Representative immune-blots for LRP-1, IR-β, LAMP-1, LC3B, PSMD4, HSP27, ATG5, and ATG3 protein expression. (D) Densitometric evaluation of the proteins listed in B, C. Band intensities were normalized to β-actin. Data represent mean ± SD of 3 independent experiments performed in duplicates. Statistically significant differences were calculated using one-way ANOVA, followed by Dunnett's post-hoc test (*p < 0.05, **p < 0.01, and ***p < 0.001) and unpaired students t-test ($p < 0.05). (E) Cells were treated with Aβ1–40 peptide with or without chloroquine (30 μM) and MG132 (20 μM) for 6 h, 4% PFA fixed and immune-fluorescently stained with LAMP-1 (green; a–d), IR-β (red; e–h), LRP-1 (red; i–l) and LC3B (green; m–p). Nuclear staining (DAPI) in blue. (For interpretation of the references to colour in this figure, the reader is referred to the web version of this article.)