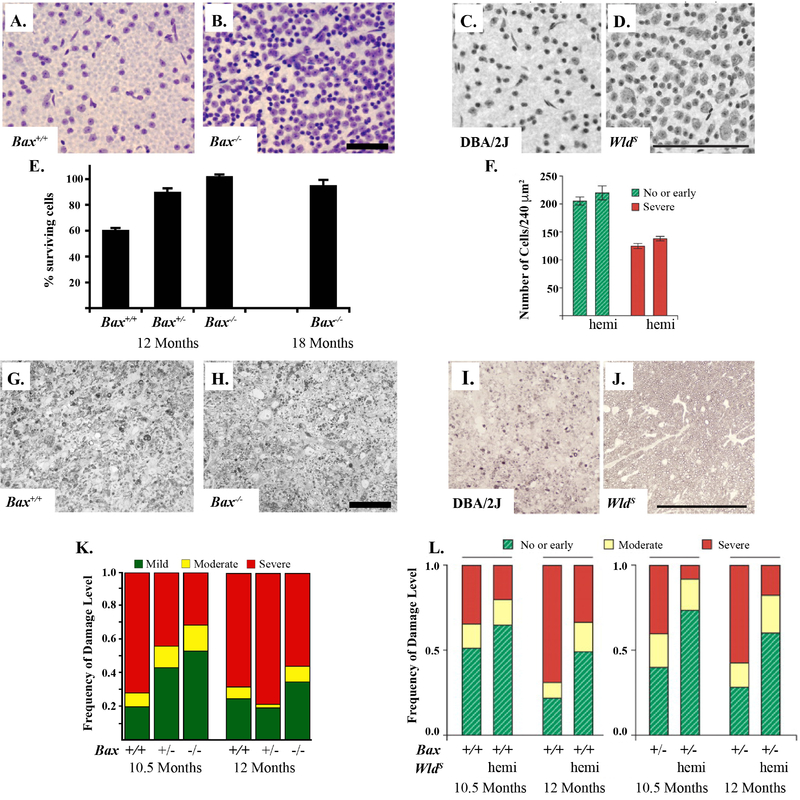
Figure 1: BAX deficiency protects RGC somas from glaucomatous damage and the WldS allele protects both somas and axons.
BAX deficiency protects RGC somas in the DBA/2J model of ocular hypertension. The corresponding retinas of severely glaucomatous optic nerves (95% or more axonal degeneration) from BAX deficient mice (Bax−/−) had significantly more RGCs as compared to wildtype animals (Bax+/+, A and B, Nissel-stained with cresyl violet, Scale bar = 50 μm). BAX deficient animals have significantly increased RGCs at baseline (BAX is required for normal developmental RGC apoptosis) and thus quantification is presented as percent surviving RGCs (retinas from corresponding optic nerves with severely glaucomatous retinas as compared to retinas with corresponding optic nerves with no glaucomatous damage from the same genotype, E). Optic nerve cross-sections stained with p-phenylenediamine (PPD) demonstrate glial scarring and loss of axons in both wildtype and BAX deficient animals (G and H, Scale bar = 50 μm). BAX deficiency does not prevent glaucomatous axonal degeneration (K). The WldS allele protects both axons and somas from glaucomatous damage. By rescuing approximately 50% of optic nerves from severe glaucoma, the WldS allele also protected RGCs in the corresponding retinas of these nerves (C- severe glaucoma wildtype DBA/2J and D- no/early glaucoma with WldS allele, Scale bar = 100 μm). In retinas with corresponding severe nerves, the WldS allele did not protect RGCs somas (F). As compared with wildtype animals, optic nerves of animals carrying the WldS allele (hemi) had less glaucomatous damage as compared to wildtype animals. Optic nerve cross-sections stained with PPD demonstrate glial scarring and loss of axons in the wildtype glaucomatous DBA/2J nerves but not in those carrying the WldS allele (I and J, Scale bar = 100 μm). BAX heterozygotes (Bax+/−) were not significantly different from wildtype DBA/2J animals (L, Figure adapted from Howell et al., 2007; Libby et al., 2005b).