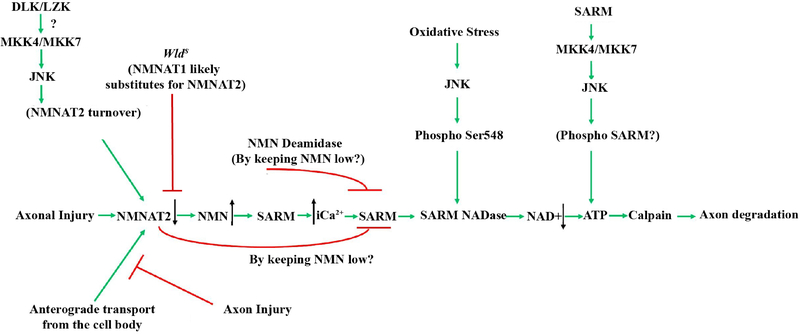
Figure 5: Axonal degeneration signaling in glaucomatous neurodegeneration.
Schematic of the molecules thought to contribute to glaucomatous neurodegeneration. Multiple possible mechanisms are thought to lead to decreased levels of NMNAT after injury. Axonal injury interrupts the anterograde transport of cytoplasmic NMNAT2 from the cell body and activation of JNK signaling leads to NMNAT2 turnover. The axonal protection afforded by animals carrying the WldS allele is thought to occur through NMNAT1 substituting for NMNAT2. Decreased levels of NMNAT2 can lead to accumulation of nicotinamide (NMN) which has been shown to be pro-degenerative at certain levels in other systems (Di Stefano et al., 2015). Nicotinamide is also a precursor of NAD+ and oral administration of nicotinamide or increasing expression of Nmnat1 (an enzyme that produces NAD+) in ocular hypertensive animals is protective from glaucomatous damage (Williams et al., 2017b). Decreased NMNAT2 activity subsequently leads to activation of SARM NADase activity likely via an increase in intracellular calcium, and additional work will be needed to determine the exact sequence of events in glaucomatous neurodegeneration given the potential complexity of NMN signaling. JNK can also activate SARM NADase activity via phosphorylation of SARM. Ultimately these events lead to deceased NAD+ levels leading to decreased ATP (which can also occur through SARM-JNK signaling), calpain activation and axon degradation. Reprinted from Biochemical Pharmacology, 161 (2019), Michael Carty and Andrew G. Bowie, SARM: from immune regulator to cell executioner, 52–62, 2019 with permission from Elsevier (Carty and Bowie, 2019).