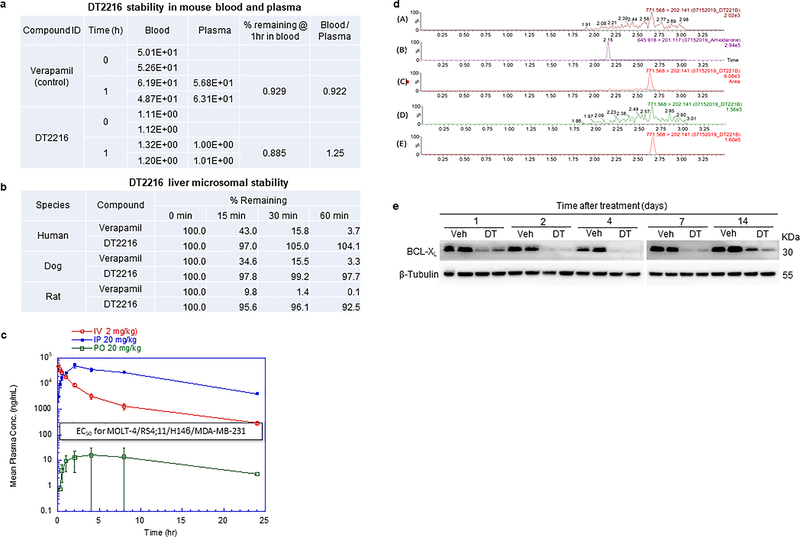
Extended Data Fig. 5. Drug metabolism and PK/PD profile of DT2216.
a, DT2216 stability in mouse blood and plasma. b, DT2216 liver microsomal stability. c, Plasma concentrations of DT2216 after a single administration of 2 mpk (i.v. injection), 20 mpk (i.p. injection) or 20 mpk (p.o. administration) are presented as Mean ± SD (n = 3 mice/group). These studies were done by BioDuro (San Diego, CA, USA), a global contract research organization, through a contract. d, MRM chromatograms of (A) DT2216 in drug-free brain homogenate, (B) Internal standard in spiked drug-free tumor homogenate (40 ng/mL), (C) DT2216 in spiked tumor homogenate (5 ng/mL; LLOQ), (D) DT2216 in tumor sample taken from vehicle dosed mouse at 24 h, (E) DT2216 in tumor sample taken at 24 h after 15 mpk/i.p. administration. e, Representative immunoblot analysis of BCL-XL in tumors at different durations after DT2216 (DT, 15 mpk/i.p.) administration (n = 2 mice in Veh and DT2216 groups at each time points). Similar results were obtained in two more immunoblot experiments. Mpk, mg/kg.