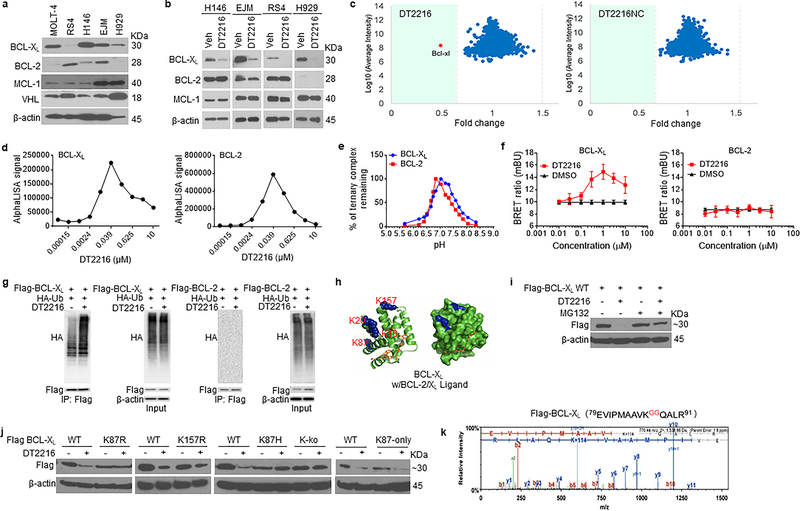
Figure 3. DT2216 is a BCL-XL-specific PROTAC and induces BCL-XL degradation through K87 ubiquitination.
a, A representative of two immunoblot analyses of BCL-XL, BCL-2, MCL-1 and VHL in distinct tumor cell lines. b, Representative immunoblot analysis of BCL-XL, BCL-2 and MCL-1 in H146 SCLC after they were treated with 0.1 μM DT2216 for 48 h and in RS4 B-ALL cells and EJM and H929 multiple myeloma cells after they were treated with 1 μM DT2216 for 16 h. Similar results were obtained in one additional independent experiment. c, Proteomic analysis showing specificity of DT2216 on BCL-XL degradation in comparison with its negative-control DT2216NC in WI-38 cells. d, Ternary complex formation of BCL-XL or BCL-2 with DT2216 and VHL determined by AlphaLISA assay. Data are expressed as mean of a single experiment (n = 2 technical replicates). Similar results were obtained in two more independent assays performed with BCL-XL. e, pH stability of ternary complex formed by DT2216 and VHL complex and BCL-XL or BCL-2 as measured by AlphaLISA assay. Data are expressed as mean (n = 2 technical replicates). Similar results were obtained in one additional independent experiment. f, NanoBRET ternary complex formation of BCL-XL and BCL-2. Ternary complex formation was determined in 293T cells after they transiently expressed HiBit-BCL-XL, LgBit, and HaloTag-VHL or HiBit-BCL-2, LgBit, and HaloTag-VHL and then treated with a serial dilution of DT2216. Data are expressed as mean ± SEM of three independent experiments. g, Representative immunoblot of HA, Flag, β-actin following Flag immunoprecipitation of protein extracts from 293T cells cotransfected as indicated with Flag-BCL-XL and HA-Ub or Flag-BCL-2 and HA-Ub plasmids, then the cells were treated with or without DT2216 (1 μM) and MG132 (10 μM) as indicated for 4 h. Data are representative of three independent experiments. h, Crystal structure of BCL-XL with ABT263 (BCL-2/XL ligand). The lysines are colored in blue. i, A representative immunoblot analysis of BCL-XL showing that DT2216 induces wild-type (WT) BCL-XL degradation in a proteasome-dependent manner. Flag-BCL-XL-WT plasmid was transfected into 293T cells for 40 h and then the cells were treated with or without DT2216 (1 μM) and MG132 (10 μM) as indicated for 6 h. Similar results were obtained in two more independent experiments. j, Representative immunoblot analysis of BCL-XL showing that DT2216 induces BCL-XL degradation dependent on K87 ubiquitination. For the analysis, Flag-BCL-XL-WT, K87R and K157R (lysine to arginine), K87H (lysine to histidine), K-ko (all the lysines in BCL-XL were mutated to arginines) and K87-only (all the lysines in BCL-XL were mutated to arginines except K87) mutant plasmids were transfected into 293T cells for 40 h, and then the cells were treated with or without 1 μM DT2216 for 6 h. Similar results were obtained in two more independent experiments. k, K87 is the only ubiquitination site triggered by DT2216. 293T cells were co-transfected with Flag-BCL-XL and HA-Ub vectors. Extracts were immunoprecipitated with anti-Flag Affinity Resin, followed by trypsin and AspN digestion and tandem mass spectrometry, as described in Material and methods. A fragmentation spectrum of ubiquitinated EVIPMAAVkQALR peptide (ubiquitinated K87 residue) of BCL-XL. Parent ion corresponding to this peptide has been subjected to higher-energy collisional dissociation in mass spectrometer. The detected b- and y-fragment ion series have been labeled. The results were obtained from a single experiment. β-actin was used as equal loading control in immunoblotting experiments shown in Fig. 3a, b, h, i and j. The uncropped immunoblot images related to this figure are provided in separate source data file.