Abstract
Background
Blood pressure (BP) guidelines for patients with aortic stenosis or a history of aortic stenosis treated with aortic valve replacement (AVR) match those in the general population, but this extrapolation may not be warranted.
Methods and Results
Among patients enrolled in the Medtronic intermediate, high, and extreme risk trials, we included those with a transcatheter AVR (n=1794) or surgical AVR (n=1103) who were alive at 30 days. The associations between early (average of discharge and 30 day post‐AVR) systolic BP (SBP) and diastolic BP (DBP) measurements and clinical outcomes between 30 days and 1 year were evaluated. Among 2897 patients, after adjustment, spline curves demonstrated an association between lower SBP (<120 mm Hg, representing 21% of patients) and DBP (<60 mm Hg, representing 30% of patients) and increased all‐cause and cardiovascular mortality and repeat hospitalization. These relationships were unchanged when patients with moderate‐to‐severe aortic regurgitation post‐AVR were excluded. After adjustment, compared with DBP 60 to <80 mm Hg, DBP 30 to <60 mm Hg was associated with increased all‐cause (adjusted hazard ratio 1.62, 95% CI 1.23–2.14) and cardiovascular mortality (adjusted hazard ratio 2.13, 95% CI 1.52–3.00), but DBP 80 to <100 mm Hg was not. Similarly, after adjustment, compared with SBP 120 to <150 mm Hg, SBP 90 to <120 mm Hg was associated with increased all‐cause (adjusted hazard ratio 1.63, 95% CI 1.21–2.21) and cardiovascular mortality (adjusted hazard ratio 1.81, 95% CI 1.25–2.61), but SBP 150 to <180 mm Hg was not.
Conclusions
Lower BP in the first month after transcatheter AVR or surgical AVR is common and associated with increased mortality and repeat hospitalization. Clarifying optimal BP targets in these patients ought to be a priority and may improve patient outcomes.
Clinical Trial Registration Information
URL: http://www.clinicaltrials.gov. Unique identifiers: NCT01586910, NCT01240902.
Keywords: aortic valve stenosis, blood pressure, mortality, transcatheter aortic valve implantation
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cardiovascular Surgery, Catheter-Based Coronary and Valvular Interventions, Stenosis
Clinical Perspective
What Is New?
Blood pressure guidelines for patients with aortic stenosis (before valve replacement) or with a history of aortic stenosis after valve replacement match those for the general population, but little research has been done to evaluate whether the distinct characteristics of these patients warrant different blood pressure targets.
This is the second moderately large study to demonstrate an adverse association between lower systolic and diastolic blood pressure values early after valve replacement for aortic stenosis and increased subsequent mortality. Specifically, systolic blood pressure values <120 to 130 mm Hg, which are currently targeted in clinical practice, were associated with harm.
What Are the Clinical Implications?
Given that this is a retrospective analysis with limited blood pressure measurements and a lack of rigorous medication tracking, a randomized study appears to be warranted to evaluate whether patients with lower blood pressure after valve replacement would benefit from efforts to raise their blood pressure.
More research is needed to better understand the factors that contribute to blood pressure and other vascular properties early after valve replacement; and the relative contribution of diastolic blood pressure versus systolic blood pressure to clinical outcomes.
Introduction
For patients with aortic stenosis (AS), the American and European guidelines for the management of patients with valvular heart disease recommend treating hypertension according to standard blood pressure guidelines.1, 2 In patients with a history of AS who have undergone aortic valve replacement (AVR), the valve guidelines provide no specific recommendations for blood pressure management.1, 2
Accordingly, the treatment of blood pressure in patients with AS or in patients with a history of AS treated with AVR matches those in the general population.3 The recent American College of Cardiology/American Heart Association blood pressure guidelines define normal blood pressure as a systolic blood pressure (SBP) <120 mm Hg and diastolic blood pressure (DBP) <80 mm Hg.3 Prescription of blood pressure‐lowering medication is recommended for patients with stage I hypertension (SBP 130–139 mm Hg or DBP 80–89 mm Hg) and with a prior cardiovascular event or at high risk for one. These criteria would apply to most patients with AS before and after AVR.3 Other large meta‐analyses recommend a target SBP <130 mm Hg as well.4, 5 The SPRINT (Systolic Blood Pressure Intervention Trial) showed that targeting an SBP <120 mm Hg yields better clinical outcomes than a target of <140 mm Hg, a finding which was also observed in patients aged >75 years regardless of their relative fitness or frailty.6, 7 In patients treated with AVR, it would seem advantageous to try to treat hypertension to optimize unloading of the left ventricle as an adjunct to unloading from valve replacement.8 Furthermore, several retrospective observational studies have shown improved long‐term clinical outcomes for patients taking inhibitors of the renin‐angiotensin system after AVR.9, 10, 11 However, we recently observed that lower SBP (SBP 100–129 mm Hg compared with SBP 130–170 mm Hg) after transcatheter AVR (TAVR) was associated with increased mortality, even after multivariable adjustment, raising concern about optimal blood pressure targets after valve replacement in patients with AS.12
In the present study, we aimed to clarify the relationship between blood pressure and clinical outcomes in a large group of patients with AS treated with TAVR or surgical AVR (SAVR). We analyzed the relationships between SBP and DBP and clinical outcomes separately and looked for thresholds below or above which risk increased. We hypothesized that lower SBP and DBP measurements, including levels commonly targeted in blood pressure guidelines, would be associated with increased mortality. These findings could have important implications for establishing specific blood pressure targets in patients with AS treated with AVR.
Methods
The data, analytic methods, and study materials are owned by the sponsor and will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
Among 3033 patients with severe, symptomatic AS at intermediate, high, or extreme surgical risk who underwent SAVR or TAVR in a previously published clinical trial, 2897 patients with a SBP recorded at discharge or 30 days were included in this analysis. There were no patients alive at 30 days without a discharge or 30‐day SBP. Patients were excluded for: an early post‐AVR SBP (average of the discharge and 30‐day measurements) of <90 mm Hg (n=2) or ≥180 mm Hg (n=14); or death before 30 days (n=113); or withdrawal or loss to follow‐up (n=7). The design, inclusion and exclusion criteria, definitions for clinical variables, and primary results of the extreme, high risk, and intermediate risk trials including patients considered for this analysis have been reported.13, 14, 15 The study protocols were approved by the institutional review board at each enrolling site, and all patients provided written informed consent.
Blood Pressure, Vascular Parameters, and Echocardiography
Heart rate and blood pressure measurements were obtained before AVR at a baseline clinical visit and after AVR at discharge and 30 days. Early post‐AVR measurements reflect an average of discharge and 30‐day values when both were available (96% of patients) or only one of them. Mean arterial pressure was calculated as: diastolic blood pressure+(1/3×pulse pressure).3 Systemic arterial compliance, systemic vascular resistance, and valvuloarterial impedance were calculated as described previously.12 These parameters, which required measurements from an echocardiogram, were obtained before AVR using measurements from the baseline echocardiogram and after AVR using measurements from the discharge echocardiogram. All echocardiograms were evaluated and measurements made by a core laboratory.
Clinical End Points
Our primary end point was all‐cause death between 30 days and 1 year after AVR. Secondary end points included cardiovascular death, non‐cardiovascular death, aortic valve‐related hospitalizations, stroke, and myocardial infarction between 30 days and 1 year and Kansas City Cardiomyopathy Questionnaire overall summary score, left ventricular (LV) mass index, LV ejection fraction (LVEF), and 6‐minute walk distance 1 year after AVR.
Statistical Analysis
Continuous variables are summarized as mean±SD and were compared between groups using the independent samples t‐test or Mann‐Whitney U‐test for skew‐distributed data. Categorical variables were compared between groups with the Chi‐square or Fisher exact test, as appropriate. The relationships between early post‐AVR SBP and DBP and clinical variables were graphically examined using scatterplots and calculation of the Pearson sample correlation coefficient.
To evaluate the associations between blood pressure and clinical outcomes, early post‐AVR SBP and DBP were analyzed separately using continuous and categorical BP variables. In both cases, univariable and multivariable Cox proportional hazards regression models were fitted. For the continuous case, BP was included in the model via a restricted cubic spline (with 5 knots placed at 5 evenly spaced percentiles of the BP variable, namely the 16.6th, 33.3th, 50th, 66.6th, and 83.3th percentiles) transformation, which was used to account for a potential non‐linear association between blood pressure and log‐hazard. For graphical inspection, reference values of 130 mm Hg (SBP) and 70 mm Hg (DBP) were used. Linearity between BP and log‐hazard of the outcome was statistically tested using a test described by Harrell.16 Sensitivity analyses were performed excluding patients with moderate or severe aortic regurgitation (AR) on their discharge echocardiogram.
For the categorical case, early post‐AVR DBP was categorized as: 30 to <60; 60 to <80 (referent); and 80 to <100 mm Hg, and early post‐AVR SBP was categorized as: 90 to <120; 120 to <150 (referent); and 150 to <180 mm Hg. Cox proportional hazards models were also used to to test for interactions between each adjustment covariate (listed below) and BP category, and those with interactions meeting P<0.05 further assessed and the corresponding subgroup estimated hazard ratios reported. In addition, survival estimates for time‐to‐event outcomes, based on all available follow‐up data, were estimated using the Kaplan–Meier method, and were compared between BP categories (either increments of 10 mm Hg or the aforementioned specified categories) using the log‐rank test.
For continuous outcomes (Kansas City Cardiomyopathy Questionnaire, LV mass index, LVEF, and 6‐minute walk distance), the mean difference from the referent category was estimated using multivariable linear regression modeling, with adjustment made for the baseline measurement of the variable of interest.
To identify variables for adjustment, we considered 41 baseline clinical factors, discharge medications, discharge echocardiographic parameters, and post‐procedure complications occurring until 30 days (Table S1). Those factors with a univariable association (P<0.10) with mortality between 30 days and 1 year were considered for stepwise selection (entry/stay criteria of 0.1/0.1) to identify a parsimonious set of covariates to include in our multivariable spline and categorical models: type of treatment (TAVR versus SAVR) (forced into the adjustment models), body mass index, New York Heart Association (III/IV versus I/II), peripheral vascular disease, use of home oxygen, prior atrial fibrillation/flutter, liver cirrhosis, immunosuppressive therapy, 5‐m gait speed, whether the patient lived independently, early (between the procedure and 30 days) stroke (any), early life threatening or disabling or major bleed, early acute kidney injury, early myocardial infarction (Table S2).
All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient Population
Among 2897 patients included in this analysis with severe, symptomatic AS at intermediate, high, or extreme surgical risk receiving SAVR or TAVR, 578 were treated in the pivotal extreme risk trial, 706 were treated in the pivotal high‐risk randomized trial, and 1613 were treated in the pivotal intermediate risk trial. Table 1 shows baseline characteristics and correlations with early post‐AVR SBP and DBP for the whole population and the patients undergoing TAVR and SAVR. The correlation between discharge and 30‐day SBP was 0.24 and the correlation between discharge and 30‐day DBP was 0.23. The clinical characteristics were as expected for this patient population. Although there are statistically significant correlations between many of the parameters and early post‐AVR SBP and DBP, these correlations are uniformly weak. Interestingly, the correlation between pre‐AVR SBP and early post‐AVR SBP is 0.32 and the correlation between pre‐AVR DBP and early post‐AVR DBP is 0.28. Table 2 shows data on post‐AVR vascular and echocardiographic parameters. While there are some expected correlations between early post‐AVR SBP and DBP with other vascular parameters, the correlations with echocardiographic parameters are notably weak. The correlation between early post‐AVR SBP and DBP was 0.35. SBP and DBP are reported for pre‐AVR, discharge, and 30‐day time points in Table S3.
Table 1.
Baseline Characteristics and Associations With Early Post‐AVR Systolic and Diastolic Blood Pressures
| All Patients (n=2897) | Correlation (r) With Early Post‐AVR SBP | Correlation (r) With Early Post‐AVR DBP | TAVR (n=1794) | SAVR (n=1103) | |
|---|---|---|---|---|---|
| Clinical | |||||
| Age, y | 81±7 | 0.06* | −0.05* | 81±7 | 81±6 |
| Women, % | 46 | 0.11* | −0.00 | 47 | 45 |
| Body mass index, kg/m2 | 29±6 | 0.00 | 0.02 | 29±6 | 29±6 |
| NYHA class III/IV, % | 72 | −0.06* | −0.11* | 76 | 67 |
| STS score | 6.3±3.9 | 0.03 | −0.13* | 6.9±4.4 | 5.4±2.6 |
| Diabetes mellitus, % | 37 | 0.03 | −0.05* | 36 | 38 |
| Creatinine >2 mg/dL, % | 3 | 0.04* | 0.00 | 3 | 3 |
| History of hypertension, % | 93 | 0.08* | −0.01 | 93 | 92 |
| Peripheral vascular disease, % | 35 | 0.02 | −0.04* | 36 | 33 |
| Prior stroke, % | 10 | −0.05* | −0.01 | 10 | 9 |
| COPD, % | 43 | −0.03 | −0.01 | 46 | 37 |
| Home oxygen, % | 11 | −0.07* | −0.09* | 14 | 5 |
| Coronary artery disease, % | 70 | −0.03 | −0.08* | 71 | 68 |
| Previous myocardial infarction, % | 20 | −0.07* | −0.03 | 22 | 18 |
| Atrial fibrillation/flutter, % | 35 | −0.11* | −0.00 | 37 | 32 |
| Cirrhosis, % | 1.3 | −0.05* | −0.02 | 1.6 | 1.0 |
| Immunosuppressive therapy, % | 10 | −0.02 | −0.01 | 10 | 9 |
| Frailty | |||||
| BMI <21 kg/m2 | 4.7 | −0.01 | 0.02 | 5.6 | 3.3 |
| 5‐m gait speed (seconds) | 8.8±12.4 | −0.03 | −0.04* | 9.5±15.3 | 7.7±5.1 |
| Grip strength < frail threshold | 66 | −0.03 | −0.06* | 67 | 65 |
| MMSE score | 27±3 | 0.01 | 0.08* | 27±3 | 27±3 |
| Wheelchair bound | 4.5 | 0.02 | −0.05* | 5.9 | 2.3 |
| ADLs (# independent) | 5.8±0.8 | −0.00 | 0.06* | 5.7±0.9 | 5.9±0.6 |
| CT measurements | |||||
| Ascending aortic dimension, mm | 33±3 | −0.04* | 0.04 | 33±3 | 33±3 |
| Sinotubular junction dimension, mm | 28±3 | −0.07* | 0.04 | 28±3 | 28±3 |
| Sinuses of Valsalva width, mm | 32±5 | −0.07* | 0.03 | 32±5 | 32±3 |
| Pre‐AVR vascular parameters | |||||
| Heart rate, bpm | 72±12 | −0.10* | 0.01 | 72±12 | 71±13 |
| Systolic blood pressure, mm Hg | 136±20 | 0.32* | 0.14* | 134±19 | 138±21 |
| Diastolic blood pressure, mm Hg | 69±11 | 0.07* | 0.28* | 68±10 | 71±11 |
| Mean arterial pressure, mm Hg | 91±12 | 0.22* | 0.25* | 90±11 | 93±13 |
| Pulse pressure, mm Hg | 67±18 | 0.31* | −0.01 | 66±18 | 67±19 |
| Systemic arterial compliance | 0.7±0.3 | −0.14* | −0.00 | 0.7±0.3 | 0.7±0.3 |
| Systemic vascular resistance | 2902±935 | 0.00 | 0.04 | 2896±954 | 2908±911 |
| Valvuloarterial impedance | 4.7±1.4 | 0.04 | 0.10* | 4.7±1.4 | 4.8±1.4 |
| Valves implanted | |||||
| Size of SAVR valve | ··· | −0.11* | 0.02 | ··· | 22.9±2.1 |
| Type of TAVR valve (CoreValve %) | ··· | 0.04 | 0.04 | 92 | ··· |
| Size of TAVR valve | ··· | −0.08* | 0.06* | 28.5±1.9 | ··· |
Data shown as percentage or mean±SD. ADL indicates activities of daily living; AVR, aortic valve replacement; BMI, body mass index; COPD, chronic obstructive pulmonary disease; MMSE, mini‐mental status examination; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement.
P<0.05.
Table 2.
Post‐AVR Vascular and Echocardiographic Characteristics and Associations With Early Post‐AVR Systolic and Diastolic Blood Pressures
| All Patients (n=2897) | Correlation (r) With Early Post‐AVR SBP | Correlation (r) With Early Post‐AVR DBP | TAVR (n=1794) | SAVR (n=1103) | |
|---|---|---|---|---|---|
| Post‐AVR vascular parameters | |||||
| Heart rate, bpm | 75±11 | −0.12* | 0.15* | 74±10 | 77±11 |
| Systolic blood pressure, mm Hg | 132±15 | ··· | 0.35* | 134±15 | 130±15 |
| Diastolic blood pressure, mm Hg | 64±9 | 0.35* | ··· | 63±9 | 66±9 |
| Mean arterial pressure, mm Hg | 87±9 | 0.79* | 0.85* | 87±9 | 87±9 |
| Pulse pressure, mm Hg | 68±15 | 0.82* | −0.25* | 71±15 | 63±14 |
| Systemic arterial compliance (discharge) | 0.6±0.2 | −0.35* | 0.13* | 0.6±0.2 | 0.6±0.3 |
| Systemic vascular resistance (discharge) | 2591±876 | 0.13* | 0.29* | 2565±861 | 2654±908 |
| Valvuloarterial impedance (discharge) | 3.9±1.3 | 0.17* | 0.19* | 3.9±1.2 | 4.1±1.3 |
| Post‐AVR echocardiographic parameters | |||||
| LVEF, % | 60±11 | 0.17* | −0.00 | 60±11 | 60±11 |
| Stroke volume index | 38±11 | 0.13* | −0.10* | 39±11 | 37±11 |
| LV end‐diastolic diameter | 49±7 | −0.02 | −0.06* | 49±7 | 47±7 |
| LV end‐systolic diameter | 32±7 | −0.12* | −0.01 | 31±7 | 32±8 |
| LV mass index | 113±33 | 0.04 | −0.07* | 116±34 | 106±30 |
| Effective orifice area index | 1.0±0.3 | 0.02 | −0.02 | 1.1±0.3 | 0.9±0.3 |
| Transvalvular mean gradient | 11±5 | 0.03 | 0.03 | 9±4 | 13±6 |
| Moderate or severe aortic regurgitation, % | 5.0 | −0.00 | −0.10* | 7.4 | 0.9 |
| Moderate or severe mitral regurgitation, % | 6.5 | −0.02 | −0.10* | 8.3 | 3.5 |
| E/e’ | 19±8 | 0.04* | −0.06* | 20±9 | 19±8 |
Data shown as percentage or mean±SD. AVR indicates aortic valve replacement; E/e’, Mitral inflow E wave velocity/tissue Doppler e’; LVEF, left ventricular ejection fraction; LV, left ventricular; SAVR, surgical aortic valve replacement, TAVR, transcatheter aortic valve replacement.
P<0.05.
Early Post‐AVR Blood Pressure, Mortality, and Hospitalizations
Kaplan Meier estimates for 30 day to 1 year all‐cause and cardiovascular mortality are shown for DBP and SBP in increments of 10 mmg for all patients and then the subgroups treated with TAVR or SAVR (Tables S4 and S5). Adjusted restricted cubic spline curves demonstrate the relationship between early post‐AVR DBP and SBP and 30 day to 1 year all‐cause and cardiovascular mortality (Figures 1A, 1B and 2A, 2B). In each case, the relationship is non‐linear. Below the cutoff value of ≈60 mm Hg, lower DBP was associated with a steady increase in all‐cause and cardiovascular mortality as DBP decreased, whereas higher DBP (60–100 mm Hg) was not associated with increased mortality. Similarly, lower SBP, ≈ <120 mm Hg, was associated with an increase in all‐cause and cardiovascular mortality as SBP decreased, whereas higher SBP (120–180 mm Hg) was not associated with increased mortality. In a sensitivity analysis excluding patients (n=128) with moderate‐to‐severe aortic regurgitation on their discharge echocardiogram, these relationships and approximate cutoff values were essentially unchanged (Figure S1). In contrast, there was no significant association between early post‐AVR DBP or SBP and 30‐day to 1‐year non‐cardiovascular mortality (Figures 1C and 2C). With respect to repeat aortic valve‐related hospitalization, an early post‐AVR DBP <60 to 65 mm Hg and early post‐AVR SBP <120 mm Hg were associated with increased risk (Figures 1D and 2D).
Figure 1.
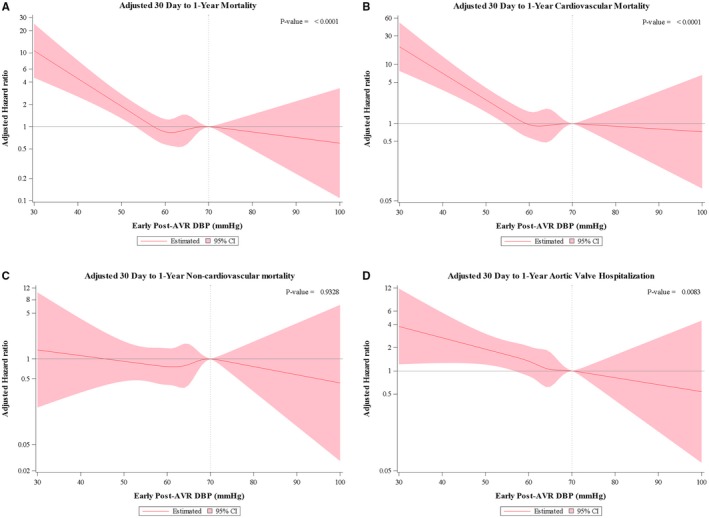
Post‐aortic valve replacement diastolic blood pressure and outcomes. Cox proportional hazard models were performed using restricted cubic splines technique. The association between early post‐aortic valve replacement diastolic blood pressure and all‐cause (A), cardiovascular (B), and non‐cardiovascular (C) 30‐day to 1‐year mortality are shown as well as the association with aortic valve‐related hospitalization (D) between 30 days and 1 year. Adjustment was made for: transcatheter aortic valve replacement (vs surgical aortic valve replacement), body mass index, New York Heart Association (III/IV vs I/II), peripheral vascular disease, home oxygen use, prior atrial fibrillation/flutter, liver cirrhosis, immunosuppressive therapy, 5 m gait speed, independent living, early stroke, early life threatening or disabling or major bleed, acute kidney injury, and early myocardial infarction. AVR indicates aortic valve replacement; DBP, diastolic blood pressure.
Figure 2.
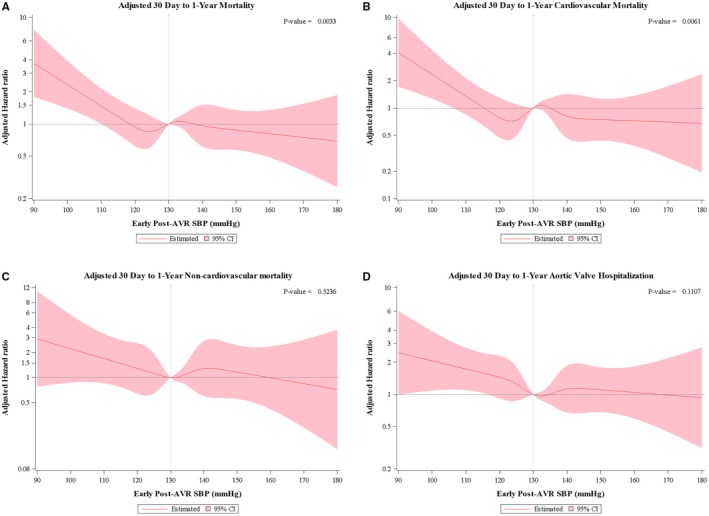
Post‐aortic valve replacement systolic blood pressure and outcomes. Cox proportional hazard models were performed using restricted cubic splines technique. The association between early post‐aortic valve replacement systolic blood pressure and all‐cause (A), cardiovascular (B), and non‐cardiovascular (C) 30‐day to 1‐year mortality are shown as well as the association with aortic valve‐related hospitalization (D) between 30 days and 1 year. Adjustment was made for the same variables as in Figure 1. AVR indicates aortic valve replacement; SBP, systolic blood pressure.
In categorical analyses, after adjustment and compared with an early post‐AVR DBP 60 to <80 mm Hg, a DBP of 30 to <60 mm Hg was associated with increased all‐cause (adjusted hazard ratio 1.62, 95% CI 1.23–2.14) and cardiovascular mortality (adjusted hazard ratio 2.13, 95% CI 1.52–3.00), but DBP 80 to <100 mm Hg was not (Table 3). An early post‐AVR DBP <60 mm Hg was observed in 867 (30%) patients. After adjustment and compared with an early post‐AVR SBP 120 to <150 mm Hg, an SBP of 90 to <120 mm Hg was associated with increased all‐cause (adjusted hazard ratio 1.63, 95% CI 1.21–2.21) and cardiovascular mortality (adjusted hazard ratio 1.81, 95% CI 1.25–2.61), but SBP 150 to <180 mm Hg was not (Table 3). An early post‐AVR SBP <120 mm Hg was observed in 622 (21%) of patients. When early post‐AVR DBP and SBP were both included in the models, the association between a lower DBP and increased all‐cause and cardiovascular mortality persisted, whereas the association between lower SBP and increased mortality was attenuated (Table S6). The association between lower early post‐AVR DBP and SBP and increased mortality was consistent across multiple sub‐groups, including those defined by type of procedure (TAVR versus SAVR), age, sex, patient risk, severity of frailty, number of antihypertensive medications, severity of AR, LV function, aortic dimensions, and valve type and size (Tables S7–S10).
Table 3.
Clinical Outcomes According to Early* Post‐AVR Diastolic Blood Pressure and Systolic Blood Pressure (All TAVR and SAVR Patients)
| Early Post‐AVR Diastolic Blood Pressure | ||||||
|---|---|---|---|---|---|---|
| Early Post‐AVR DBP (mm Hg) | Adjusted† HR (95% CI) for Each Group Compared With Referent 60 to <80 mm Hg | |||||
| 30 D to 1 Y All‐Cause Mortality | 30 D to 1 Y Cardiovascular Mortality | 30 D to 1 Y Non‐Cardiovascular Mortality | 30 D to 1 Y Aortic Valve Hospitalization | 30 D to 1 Y Stroke (All) | 30 D to 1 Y Myocardial Infarction (Spontaneous) | |
| 30 to <60 | 1.62 (1.23–2.14) | 2.13 (1.52–3.00) | 0.94 (0.57–1.55) | 1.58 (1.17–2.15) | 1.22 (0.72–2.07) | 1.33 (0.49–3.64) |
| 60 to <80 (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 80 to <100 | 1.18 (0.57–2.43) | 0.79 (0.25–2.54) | 1.65 (0.65–4.20) | 1.08 (0.47–2.48) | 0.42 (0.06–3.05) | 3.09 (0.68–14.13) |
| Early Post‐AVR Systolic Blood Pressure | ||||||
|---|---|---|---|---|---|---|
| Early Post‐AVR SBP (mm Hg) | Adjusted† HR (95% CI) for Each Group Compared With Referent 120 to <150 mm Hg | |||||
| 30 D to 1 Y All‐Cause Mortality | 30 D to 1 Y Cardiovascular Mortality | 30 D to 1 Y Non‐Cardiovascular Mortality | 30 D to 1 Y Aortic Valve Hospitalization | 30 D to 1 Y Stroke (All) | 30 D to 1 Y Myocardial Infarction (Spontaneous) | |
| 90 to <120 | 1.63 (1.21–2.21) | 1.81 (1.25–2.61) | 1.33 (0.79–2.24) | 1.50 (1.07–2.12) | 1.03 (0.55–1.91) | 2.15 (0.73–6.34) |
| 120 to <150 (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 150 to <180 | 1.05 (0.67–1.64) | 1.03 (0.58–1.83) | 1.07 (0.52–2.20) | 0.83 (0.49–1.38) | 1.19 (0.57–2.46) | 1.16 (0.32–4.19) |
AVR indicates aortic valve replacement; DBP, diastolic blood pressure; SAVR, surgical aortic valve replacement; SBP, systolic blood pressure; TAVR, transcatheter aortic valve replacement.
Early was the period between the procedure and 30 days.
Adjusted for TAVR (vs SAVR), body mass index, New York Heart Association (III/IV vs I/II), peripheral vascular disease, on home oxygen, prior atrial fibrillation/flutter, liver cirrhosis, immunosuppressive therapy, 5‐meter gait speed, does not live independently, early stroke (any), early life threatening or disabling or major bleed, early acute kidney injury, early myocardial infarction.
When looking at groups defined by an early post‐AVR DBP <60 versus ≥60 mm Hg and SBP <120 versus ≥120 mm Hg, a DBP <60 mm Hg appeared to have more of an adverse influence than an SBP <120 mm Hg with the highest mortality observed in those with a DBP <60 mm Hg and SBP <120 mm Hg (Figure 3). We also evaluated 30‐day to 1‐year all‐cause and cardiovascular mortality for groups defined by a discharge DBP <60 versus ≥60 mm Hg and 30‐day DBP <60 versus ≥60 mm Hg (Figure 4). Mortality was the highest in those with persistent DBP <60 mm Hg followed by those with a discharge DBP ≥60 mm Hg but then a 30‐day DBP <60 mm Hg. Mortality was similar in those with a 30‐day DBP ≥60 mm Hg regardless of their discharge DBP. When similar analyses were performed based on discharge and 30‐day SBP, the relationships seen for DBP were not as apparent, although an SBP <120 mm Hg at 30 days, regardless of discharge SBP, tended to be associated with the highest all‐cause mortality rates (Figure S2).
Figure 3.
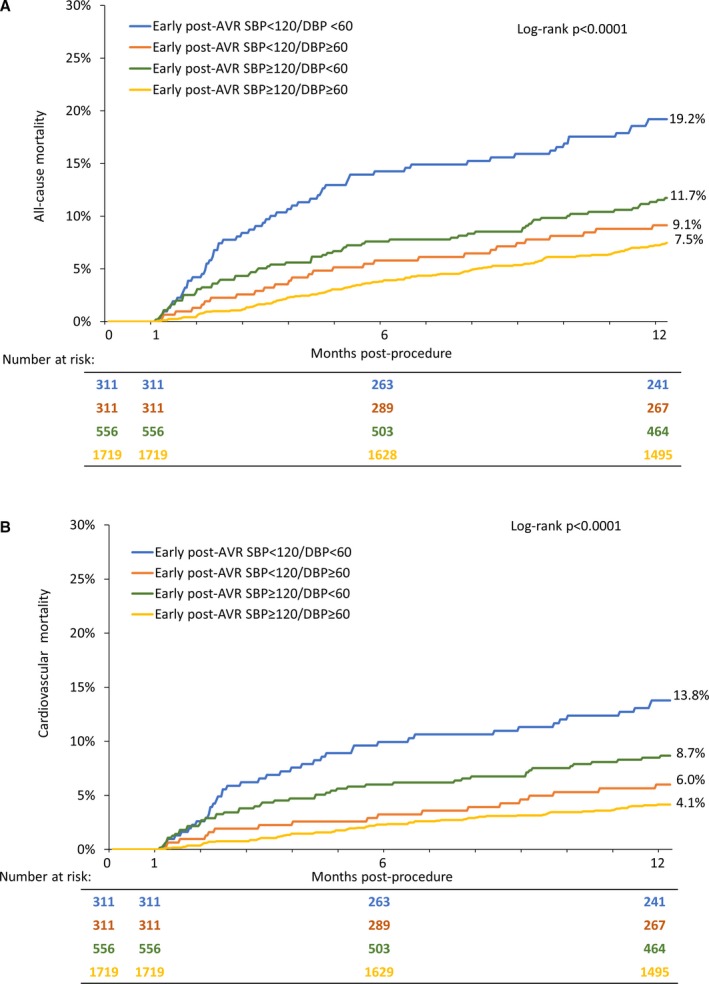
Post‐aortic valve replacement systolic and diastolic blood pressure and outcomes. Kaplan–Meier curves are shown for all‐cause (A) and cardiovascular (B) mortality between 30 days and 1 year for early post‐aortic valve replacement blood pressure groups defined by systolic blood pressure ≥120 vs <120 mm Hg and diastolic blood pressure ≥60 vs <60 mm Hg. AVR indicates aortic valve replacement; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Figure 4.
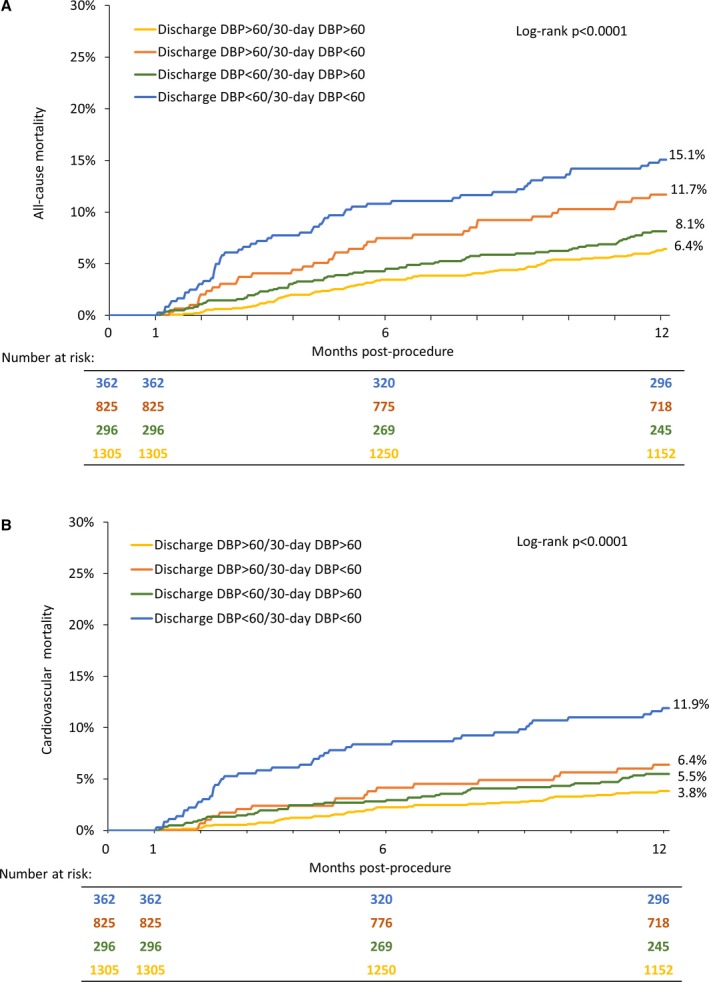
Discharge and 30‐day diastolic blood pressure and outcomes. Kaplan–Meier curves are shown for all‐cause (A) and cardiovascular (B) mortality between 30 days and 1 year for diastolic blood pressure groups defined by discharge diastolic blood pressure ≥60 vs <60 mm Hg and 30‐day diastolic blood pressure ≥60 vs <60 mm Hg. DBP indicates diastolic blood pressure.
Stroke and Myocardial Infarction
Adjusted restricted cubic spline curves demonstrate no significant relationships between any level of early post‐AVR DBP or SBP and the 30‐day to 1‐year hazard for stroke or myocardial infarction (Figures S3 and S4). This was corroborated by the categorical analyses shown in Table 3.
Quality of Life, Walk Distance, and the Left Ventricle
Compared with an early post‐AVR DBP 60 to <80 mm Hg and adjusted for baseline values, a DBP 30 to <60 mm Hg was associated with a shorter 6‐minute walk distance at 1 year after AVR (Table 4). No significant associations were observed between early post‐AVR DBP and quality of life, LV mass index, or LVEF at 1 year after AVR. For early post‐AVR SBP, there were no significant associations observed with 1‐year 6‐minute walk distance, quality of life, or LV mass index or LVEF (Table 4).
Table 4.
Additional 1 Year Clinical End Points According to Early Post‐AVR DBP and SBP (All TAVR and SAVR Patients)
| Early* Post‐AVR Diastolic Blood Pressure (DBP) | ||||
|---|---|---|---|---|
| Early Post‐AVR DBP (mm Hg) | Adjusted† Mean Difference (95% CI) Compared With Referent 60 to <80 mm Hg | |||
| 1 Y KCCQ (Overall Summary Score) | 1 Y 6 Min Walk Distance | 1 Y LV Mass Index | 1 Y LVEF | |
| 30 to <60 | −1.06 (−2.86, 0.74) | −14.69 (−25.56, −3.83) | 1.18 (−1.66, 4.03) | −0.07 (−0.91, 0.77) |
| 60 to <80 (reference) | ··· | ··· | ··· | ··· |
| 80 to <100 | −0.77 (−4.59, 3.05) | −1.73 (−24.85, 21.38) | −2.02 (−8.04, 4.01) | 0.72 (−1.05, 2.49) |
| Early Post‐AVR Systolic Blood Pressure (SBP) | ||||
|---|---|---|---|---|
| Early Post‐AVR SBP (mm Hg) | Adjusted* Mean Difference (95% CI) Compared With Referent 120 to <150 mm Hg | |||
| 1 Y KCCQ (Overall Summary Score) | 1 Y 6 Min Walk Distance | 1 Y LV Mass Index | 1 Y LVEF | |
| 90 to <120 | −1.57 (−3.62, 0.48) | −1.94 (−14.34, 10.47) | 1.73 (−1.54, 5.00) | −0.15 (−1.11, 0.81) |
| 120 to <150 (reference) | ··· | ··· | ··· | ··· |
| 150 to <180 | 0.00 (−2.38, 2.39) | −5.83 (−20.04 8.37) | 1.61 (−2.18, 5.40) | 0.68 (−0.45, 1.81) |
AVR indicates aortic valve replacement; DBP, diastolic blood pressure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; LVEF, left ventricular ejection fraction; SAVR, surgical aortic valve replacement; SBP, systolic blood pressure; TAVR, transcatheter aortic valve replacement.
Early was the period between the procedure and 30 days.
Adjusted for baseline value.
Medications
Among those with an early post‐AVR DBP 30 to <60 mm Hg, at least 89% and 39% were on ≥1 blood pressure‐lowering medication at discharge and 30 days, respectively, and at least 41% and 19% were on ≥2 blood pressure‐lowering medications at discharge and 30 days, respectively (Table S11). Similarly, among those with an early post‐AVR SBP 90 to <120 mm Hg, at least 87% and 43% were on ≥1 blood pressure‐lowering medication at discharge and 30 days, respectively, and at least 40% and 22% were on ≥2 blood pressure‐lowering medications at discharge and 30 days, respectively (Table S12).
Discussion
In 2897 patients with severe symptomatic AS undergoing SAVR or TAVR, we showed that lower DBP (<60 mm Hg) and SBP (<120 mm Hg) in the first month after AVR were associated with increased 30‐day to 1‐year all‐cause and cardiovascular mortality and a higher rate of hospital readmission. These associations were significant after adjustment, in sensitivity analyses excluding patients with moderate or severe aortic regurgitation, and across multiple subgroups. Between the 2, lower DBP had the stronger association with adverse outcomes. Sustained DBP <60 mm Hg (at discharge and 30 days) was associated with the highest 30‐day to 1‐year mortality. Notably, early post‐AVR DBP <60 mm Hg and SBP <120 mm Hg were common, observed in 30% and 21% of the cohort, respectively. Many patients with lower blood pressure values were taking ≥1 blood pressure lowering medications. Collectively, these data combined with other reports suggest that invigorated attention to blood pressure after AVR is warranted to optimize outcomes and that blood pressure goals in these patients may need to be higher than in the general population. These findings are particularly relevant as recent observational studies have suggested a potential benefit of inhibitors of the renin‐angiotensin system after AVR without specifically considering any lower blood pressure limits.9, 10, 11
These findings confirm and extend those recently made from the PARTNER (Placement of Aortic Transcatheter Valves) trial and other smaller studies.12, 17 In the PARTNER analysis, among patients undergoing TAVR with severe symptomatic AS at high or prohibitive risk for surgery, an SBP of 100 to 129 mm Hg compared with 130 to 170 mm Hg at 30 days after TAVR was associated with higher 30‐day to 1‐year all‐cause and cardiovascular mortality. Further, compared with an SBP of 130 to 139 mm Hg, 30‐day SBP 100 to 109, 110 to 119, and 120 to 129 mm Hg were each associated with increased mortality in adjusted analyses, whereas 30‐day SBP 140 to 149, 150 to 159, and 160 to 169 mm Hg were not. An association between lower 30‐day DBP and increased mortality was also observed, even after adjustment for 30‐day SBP and other clinical factors. The findings reported here not only confirm these prior observations in a larger study population and extend them to one including patients at intermediate risk for surgery and those undergoing SAVR, they also emphasize the importance of considering DBP and not just SBP. We also examined other relevant clinical end points, including non‐cardiovascular mortality, repeat hospitalization, stroke, and myocardial infarction and considered whether additional clinical factors (eg, frailty, valve size, TAVR versus SAVR, and aortic dimensions) might alter the relationship between blood pressure and mortality. We observed relatively modest correlations between pre‐AVR SBP and early post‐AVR SBP (0.32) and between pre‐AVR DBP and early post‐AVR DBP (0.28), emphasizing the known effects of valve replacement on the arterial tree.18 Based on the sensitivity and interaction analyses, our findings are unlikely to be confounded or altered by paravalvular AR or LV function, neither of which was associated with mortality in this cohort. While the significance of paravalvular AR may be underappreciated by transthoracic echocardiography and the mean early post‐AVR DBPs may be lower than expected, the early post‐AVR DBPs are similar to those observed in other series and unlikely to be substantially related to post‐AVR AR because those undergoing TAVR and SAVR experienced a similar 5 mm Hg drop in DBP after the procedure and significant AR occurs rarely after SAVR.12 With weak correlations between both early post‐AVR SBP and DBP and clinical, frailty, and echocardiographic parameters, it remains unclear what factors underlie a lower blood pressure after AVR.
The relationship between blood pressure and mortality is known to follow a J‐shape in the general population, particularly with respect to DBP.19, 20, 21, 22, 23 Such a relationship has also been observed for patients with asymptomatic AS before AVR.24 Lower DBP is associated with increased myocardial damage and increased angina, which may be exacerbated in patients with concomitant myocardial hypertrophy.20, 25, 26 Interestingly, the DBP cutoff below which risk increased (<60 mm Hg) was lower than observed in other populations, and there was no increase in risk associated with higher DBPs (up to 100 mm Hg). Analogously, we observed an increase in risk for early post‐AVR SBP <120 mm Hg, but not for higher SBP values (up to 180 mm Hg).
As previously speculated, these findings may be related to (1) the implanted prosthetic valve and displacement of native valve leaflets into the sinuses which may alter flow from the aorta into the coronary arteries requiring a higher aortic pressure to maintain coronary flow; (2) left ventricular hypertrophy, diastolic dysfunction, and a higher left ventricular end‐diastolic pressure which are common in patients with AS treated with valve replacement and tend to reduce coronary perfusion pressure; or other reasons.12 With data available to us from this device‐focused trial, we are unable to elucidate the mechanism(s) for our findings, which will require further studies. While it cannot be ruled out, reverse causality as an explanation for our findings is unlikely. The associations between early post‐AVR SBP and DBP and clinical factors and measures of frailty were extremely weak. Further, we adjusted for comorbidities and post‐procedural complications in our multivariable models. Finally, the associations between lower SBP and DBP and increased all‐cause mortality were driven by the association with increased cardiovascular mortality and not non‐cardiovascular mortality.
These findings have important clinical implications. There are no guidelines for blood pressure targets specifically for patients with a history of AS treated with AVR.1, 2, 3 Implicitly, then, blood pressure guidelines for the general population would apply, specifically targeting a BP <130/80 mm Hg in these patients.3 However, our data suggest that these targets could be harmful for patients who have undergone AVR for AS. Early post‐AVR SBP below 120 mm Hg is associated with an increased risk of mortality and repeat hospitalizations. Further, our data highlight the adverse association between low DBP and clinical outcomes, which is not considered in treatment algorithms that generally focus on SBP targets. Among those at increased risk for mortality with DBP 30 to <60 mm Hg (n=867), 342 (39.4%) had an SBP ≥130 mm Hg, making them candidates for initiation or intensification of antihypertensive therapy which would expose them to additional risk from an even lower DBP. We acknowledge that a SPRINT ancillary analysis showed that although patients with the lowest DBP had increased risk, the treatment benefit associated with a lower target SBP was consistent across DBP groups, including those with the lowest DBP before randomization.27 However, unlike SPRINT, in patients with AS treated with valve replacement we observed increased risk associated with the same SBP levels targeted in the intensive treatment arm.6
Our data suggest that rather than focusing on treatment of high blood pressure to reduce risk in patients treated with valve replacement for AS, we should focus attention on those with lower blood pressure and consider reducing doses or eliminating blood pressure‐lowering medications to reduce risk. Relevant to this, many patients with lower blood pressure values were on ≥1 blood pressure‐lowering medications. Further, these patients were not rare. Patients with an SBP 90 to <120 mm Hg represented 21% of the patients in this analysis and patients with SBP 100 to <130 mm Hg represented 47% of the patients in the PARTNER analysis which included those with 30‐day SBP between 100 and 170 mm Hg. Similarly, a DBP <60 mm Hg is observed in a large minority of patients recently treated with valve replacement: 30% in this cohort and 40% in the PARTNER analysis. Figure 4 shows that among patients with a discharge DBP <60 mm Hg, survival between 30 days and 1 year was better if their 30‐day DBP was ≥60 mm Hg.
Several limitations should be considered when interpreting our findings. Although this analysis averages 2 blood pressure values taken in the early post‐AVR period (discharge and 30 day), that is still a relatively limited number to characterize a patient's blood pressure. Blood pressure measurements were obtained according to the standard of care at each site and not standardized. Medication data were captured inconsistently across the trials and only β‐blockers and renin‐angiotensin system antagonists were routinely categorized, limiting our analyses on the potential relationship between blood pressure and medications. However, this limitation means that our assessment of the proportion of patients with lower blood pressure values who are on blood pressure‐lowering medications is an underestimate. The actual number of patients with low SBP or DBP measurements early post‐AVR who are on ≥1 blood pressure‐lowering medications is likely higher. Finally, this is a retrospective analysis and our findings could be influenced by unmeasured confounders.
Conclusions
we showed that lower DBP (<60 mm Hg) and lower SBP (<120 mm Hg) in the first month after TAVR or SAVR are each associated with increased all‐cause and cardiovascular mortality and repeat hospitalizations. In light of these and prior findings, a randomized strategy trial may now be appropriate in patients with AS treated with AVR to determine whether patients with lower BP measurements (SBP <120 or 130 mm Hg or DBP <60 mm Hg) should have their medication regimen adjusted to allow their BP to rise above these levels. Given the growing number of patients with AS undergoing valve replacement and the large percentage of patients with these lower BP values, clarifying BP targets and appropriate management strategies is both timely and consequential.
Sources of Funding
The CoreValve U.S. Pivotal trials and this analysis were supported by Medtronic (Minneapolis, MN).
Disclosures
Dr. Lindman has served on the scientific advisory board for Roche Diagnostics, has received research grants from Edwards Lifesciences and Roche Diagnostics, and has consulted for Medtronic. Dr. Beckman has consulted for Astra Zeneca, Bristol Myers Squibb, Boehringer Ingelheim, Merck, Novo Nordisk, and Sanofi, and has served on the Data Safety and Monitoring Board for Bayer and Novartis. Dr. Barker has served on the advisory board for Medtronic and Boston Scientific. Dr. Cavalcante has consulted for Siemens, Medtronic, and Circle Cardiovascular Imaging, and received research grants from Abbott and Medtronic. Dr. Elmariah has received institutional grant support from Edwards Lifesciences and received consulting fees from Astra Zeneca and Medtronic. Drs. Huang and Hickey are employees of Medtronic. Dr. Adams has received grant support from Medtronic and has royalty agreements through Mount Sinai School of Medicine with Medtronic and Edwards Lifesciences. Dr. Popma has received institutional grant support from Boston Scientific, Direct Flow Medical, and Medtronic; has received consultant fees from Boston Scientific and Direct Flow Medical; and owns equity in Direct Flow Medical. Dr. Reardon has received consultant fees from Medtronic paid to his department. The remaining authors have no disclosures to report.
Supporting information
Table S1. Univariable Associations With Mortality Between 30 Days and 1 Year
Table S2. Multivariable Model for Factors Associated With Mortality Between 30 Days and 1 Year*
Table S3. Blood Pressure at Baseline, Discharge, and 30 Days
Table S4. All‐Cause and Cardiovascular Mortality Rates From 30 Days to 1 Year According to Early Post‐AVR Diastolic Blood Pressure
Table S5. All‐Cause and Cardiovascular Mortality Rates From 30 Days to 1 Year According to Early Post‐AVR Systolic Blood Pressure
Table S6. Clinical Outcomes According to Early Post‐AVR DBP and SBP (All TAVR and SAVR Patients)
Table S7. All‐Cause Mortality (30 Days to 1 Year) – Sub‐group Analyses for Early Post‐AVR Diastolic Blood Pressure in the Full Cohort (TAVR+SAVR Patients)
Table S8. All‐Cause Mortality (30 days to 1 year) – Sub‐group Analyses for Early Post‐AVR Diastolic Blood Pressure in the Full Cohort (TAVR+SAVR patients). Additional Information on Significant Interactions Seen in Table S7
Table S9. All‐Cause Mortality (30 Days to 1 Year) – Sub‐Group Analyses for Early Post‐AVR Systolic Blood Pressure in the Full Cohort (TAVR+SAVR Patients)
Table S10. All‐Cause Mortality (30 Days to 1 Year) – Sub‐Group Analyses for Early Post‐AVR Systolic Blood Pressure in the Full Cohort (TAVR+SAVR Patients). Additional Information on Significant Interactions Seen in Table S9
Table S11. Blood Pressure Medication Utilization at Discharge and 30 Days Based on Early Post‐AVR Diastolic Blood Pressure
Table S12. Blood Pressure Medication Use at Discharge and 30 Days Based on Early Post‐AVR Systolic Blood Pressure
Figure S1. Post‐AVR Diastolic and systolic blood pressures and mortality (excluding patients with moderate or severe total AR on the discharge echocardiogram) (sensitivity analysis).
Figure S2. Discharge and 30‐day systolic blood pressure and outcomes.
Figure S3. Post‐AVR diastolic blood pressures and clinical outcomes.
Figure S4. Post‐AVR systolic blood pressures and clinical outcomes.
Acknowledgments
We thank Colleen Gilbert, PharmD, an employee of Medtronic, for editorial support.
(J Am Heart Assoc. 2019;8:e014020 DOI: 10.1161/JAHA.119.014020.)
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; American Collegeof Cardiology, American Heart Association Task Force on Practice G . AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484–e594. [DOI] [PubMed] [Google Scholar]
- 4. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. The Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 5. Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK, He J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta‐analysis. JAMA Cardiol. 2017;2:775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel‐Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM, SPRINT Research Group . Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindman BR, Otto CM. Time to treat hypertension in patients with aortic stenosis. Circulation. 2013;128:1281–1283. [DOI] [PubMed] [Google Scholar]
- 9. Goel SS, Aksoy O, Gupta S, Houghtaling PL, Tuzcu EM, Marwick T, Mihaljevic T, Svensson L, Blackstone EH, Griffin BP, Stewart WJ, Barzilai B, Menon V, Kapadia SR. Renin‐angiotensin system blockade therapy after surgical aortic valve replacement for severe aortic stenosis: a cohort study. Ann Intern Med. 2014;161:699–710. [DOI] [PubMed] [Google Scholar]
- 10. Inohara T, Manandhar P, Kosinski AS, Matsouaka RA, Kohsaka S, Mentz RJ, Thourani VH, Carroll JD, Kirtane AJ, Bavaria JE, Cohen DJ, Kiefer TL, Gaca JG, Kapadia SR, Peterson ED, Vemulapalli S. Association of renin‐angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter aortic valve replacement. JAMA. 2018;320:2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ochiai T, Saito S, Yamanaka F, Shishido K, Tanaka Y, Yamabe T, Shirai S, Tada N, Araki M, Naganuma T, Watanabe Y, Yamamoto M, Hayashida K. Renin‐angiotensin system blockade therapy after transcatheter aortic valve implantation. Heart. 2018;104:644–651. [DOI] [PubMed] [Google Scholar]
- 12. Lindman BR, Otto CM, Douglas PS, Hahn RT, Elmariah S, Weissman NJ, Stewart WJ, Ayele GM, Zhang F, Zajarias A, Maniar HS, Jilaihawi H, Blackstone E, Chinnakondepalli KM, Tuzcu EM, Leon MB, Pibarot P. Blood pressure and arterial load after transcatheter aortic valve replacement for aortic stenosis. Circ Cardiovasc Imaging. 2017;10:e006308. [DOI] [PubMed] [Google Scholar]
- 13. Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J Jr, Hughes GC, Harrison JK, Coselli J, Diez J, Kafi A, Schreiber T, Gleason TG, Conte J, Buchbinder M, Deeb GM, Carabello B, Serruys PW, Chenoweth S, Oh JK; CoreValve United States Clinical Investigators . Transcatheter aortic valve replacement using a self‐expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. [DOI] [PubMed] [Google Scholar]
- 14. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK; U.S. CoreValve Clinical Investigators . Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 15. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators . Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 16. Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer‐Verlag; 2010. [Google Scholar]
- 17. Perlman GY, Loncar S, Pollak A, Gilon D, Alcalai R, Planer D, Lotan C, Danenberg HD. Post‐procedural hypertension following transcatheter aortic valve implantation: incidence and clinical significance. JACC Cardiovasc Interv. 2013;6:472–478. [DOI] [PubMed] [Google Scholar]
- 18. Yotti R, Bermejo J, Gutierrez‐Ibanes E, Perez del Villar C, Mombiela T, Elizaga J, Benito Y, Gonzalez‐Mansilla A, Barrio A, Rodriguez‐Perez D, Martinez‐Legazpi P, Fernandez‐Aviles F. Systemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacement. J Am Coll Cardiol. 2015;65:423–433. [DOI] [PubMed] [Google Scholar]
- 19. Messerli FH, Panjrath GS. The J‐curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827–1834. [DOI] [PubMed] [Google Scholar]
- 20. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet. 1987;1:581–584. [DOI] [PubMed] [Google Scholar]
- 22. Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–893. [DOI] [PubMed] [Google Scholar]
- 23. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J‐curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA. 1991;265:489–495. [PubMed] [Google Scholar]
- 24. Nielsen OW, Sajadieh A, Sabbah M, Greve AM, Olsen MH, Boman K, Nienaber CA, Kesaniemi YA, Pedersen TR, Willenheimer R, Wachtell K. Assessing optimal blood pressure in patients with asymptomatic aortic valve stenosis: the SEAS Study. Circulation. 2016;134:455–468. [DOI] [PubMed] [Google Scholar]
- 25. Peri‐Okonny PA, Patel KK, Jones PG, Breeding T, Gosch KL, Spertus JA, Arnold SV. Low diastolic blood pressure is associated with angina in patients with chronic coronary artery disease. J Am Coll Cardiol. 2018;72:1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harrison DG, Florentine MS, Brooks LA, Cooper SM, Marcus ML. The effect of hypertension and left ventricular hypertrophy on the lower range of coronary autoregulation. Circulation. 1988;77:1108–1115. [DOI] [PubMed] [Google Scholar]
- 27. Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, Wei G, Campbell RC, Conroy M, Freedman BI, Haley W, Horwitz E, Kitzman D, Lash J, Papademetriou V, Pisoni R, Riessen E, Rosendorff C, Watnick SG, Whittle J, Whelton PK, SPRINT Research Group . Influence of baseline diastolic blood pressure on effects of intensive compared with standard blood pressure control. Circulation. 2018;137:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariable Associations With Mortality Between 30 Days and 1 Year
Table S2. Multivariable Model for Factors Associated With Mortality Between 30 Days and 1 Year*
Table S3. Blood Pressure at Baseline, Discharge, and 30 Days
Table S4. All‐Cause and Cardiovascular Mortality Rates From 30 Days to 1 Year According to Early Post‐AVR Diastolic Blood Pressure
Table S5. All‐Cause and Cardiovascular Mortality Rates From 30 Days to 1 Year According to Early Post‐AVR Systolic Blood Pressure
Table S6. Clinical Outcomes According to Early Post‐AVR DBP and SBP (All TAVR and SAVR Patients)
Table S7. All‐Cause Mortality (30 Days to 1 Year) – Sub‐group Analyses for Early Post‐AVR Diastolic Blood Pressure in the Full Cohort (TAVR+SAVR Patients)
Table S8. All‐Cause Mortality (30 days to 1 year) – Sub‐group Analyses for Early Post‐AVR Diastolic Blood Pressure in the Full Cohort (TAVR+SAVR patients). Additional Information on Significant Interactions Seen in Table S7
Table S9. All‐Cause Mortality (30 Days to 1 Year) – Sub‐Group Analyses for Early Post‐AVR Systolic Blood Pressure in the Full Cohort (TAVR+SAVR Patients)
Table S10. All‐Cause Mortality (30 Days to 1 Year) – Sub‐Group Analyses for Early Post‐AVR Systolic Blood Pressure in the Full Cohort (TAVR+SAVR Patients). Additional Information on Significant Interactions Seen in Table S9
Table S11. Blood Pressure Medication Utilization at Discharge and 30 Days Based on Early Post‐AVR Diastolic Blood Pressure
Table S12. Blood Pressure Medication Use at Discharge and 30 Days Based on Early Post‐AVR Systolic Blood Pressure
Figure S1. Post‐AVR Diastolic and systolic blood pressures and mortality (excluding patients with moderate or severe total AR on the discharge echocardiogram) (sensitivity analysis).
Figure S2. Discharge and 30‐day systolic blood pressure and outcomes.
Figure S3. Post‐AVR diastolic blood pressures and clinical outcomes.
Figure S4. Post‐AVR systolic blood pressures and clinical outcomes.


