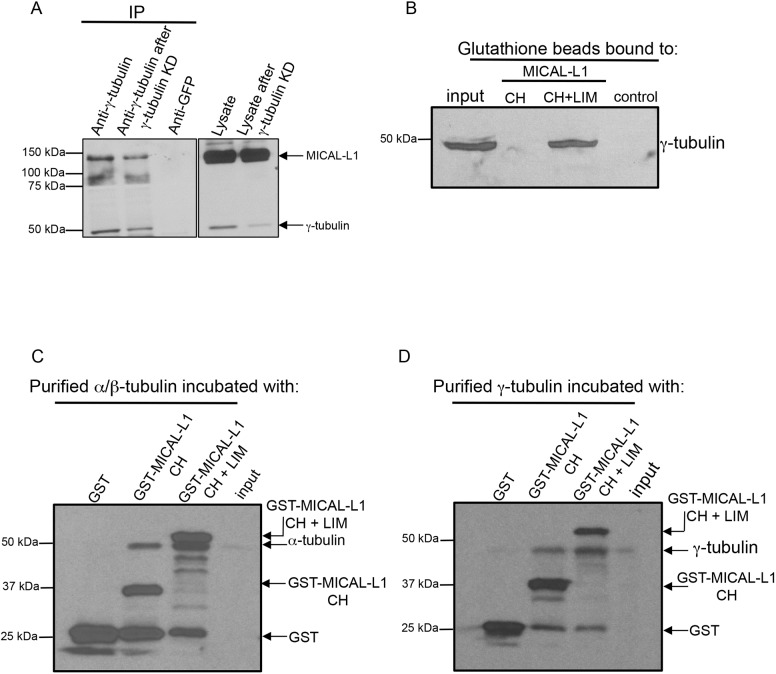
Fig. 3.
MICAL-L1 interacts directly with tubulins. (A) Mock-treated (lysate) or γ-tubulin knockdown (lysate after γ-tubulin knockdown) NIH3T3 cells were lysed and subjected to immunoblotting (right panel). Immunoprecipitations (IP) were undertaken using anti-γ-tubulin, or rabbit anti-GFP (control) antibodies. Immunoprecipitated proteins were immunoblotted with anti-MICAL-L1 and anti-γ-tubulin antibodies (left panel). (B) 500 ng of purified GST alone, GST–MICAL-L1-CH domain or GST-MICAL-L1-CH+LIM domains were bound to GST beads, and incubated with NIH3T3 cell lysates. Precipitated proteins were eluted from the beads and immunoblotted using anti-γ-tubulin antibody. (C,D) 500 ng of purified GST, GST–MICAL-L1-CH domain or GST–MICAL-L1-CH+LIM domains (bait) were bound to GST beads, and treated with 1 U of micrococcal nuclease. 0.1 µg purified α-tubulin–β-tubulin heterodimers (C) or γ-tubulin (D) were treated with 1 U of micrococcal nuclease. Immobilized GST–bait proteins were incubated with α-tubulin–β-tubulin heterodimers or γ-tubulin, and bound protein was immunoblotted.