ABSTRACT
Phosphomannomutase 2 deficiency, or PMM2-CDG, is the most common congenital disorder of glycosylation and affects over 1000 patients globally. There are no approved drugs that treat the symptoms or root cause of PMM2-CDG. To identify clinically actionable compounds that boost human PMM2 enzyme function, we performed a multispecies drug repurposing screen using a novel worm model of PMM2-CDG, followed by PMM2 enzyme functional studies in PMM2-CDG patient fibroblasts. Drug repurposing candidates from this study, and drug repurposing candidates from a previously published study using yeast models of PMM2-CDG, were tested for their effect on human PMM2 enzyme activity in PMM2-CDG fibroblasts. Of the 20 repurposing candidates discovered in the worm-based phenotypic screen, 12 were plant-based polyphenols. Insights from structure–activity relationships revealed epalrestat, the only antidiabetic aldose reductase inhibitor approved for use in humans, as a first-in-class PMM2 enzyme activator. Epalrestat increased PMM2 enzymatic activity in four PMM2-CDG patient fibroblast lines with genotypes R141H/F119L, R141H/E139K, R141H/N216I and R141H/F183S. PMM2 enzyme activity gains ranged from 30% to 400% over baseline, depending on genotype. Pharmacological inhibition of aldose reductase by epalrestat may shunt glucose from the polyol pathway to glucose-1,6-bisphosphate, which is an endogenous stabilizer and coactivator of PMM2 homodimerization. Epalrestat is a safe, oral and brain penetrant drug that was approved 27 years ago in Japan to treat diabetic neuropathy in geriatric populations. We demonstrate that epalrestat is the first small molecule activator of PMM2 enzyme activity with the potential to treat peripheral neuropathy and correct the underlying enzyme deficiency in a majority of pediatric and adult PMM2-CDG patients.
KEY WORDS: Phosphomannomutase 2 deficiency, Drug repurposing, PMM2-CDG, Congenital disorder of glycosylation, Aldose reductase inhibitor, Epalrestat
Editor's choice: Drug repurposing screens using worm and patient fibroblast models of PMM2-CDG led to the discovery of epalrestat, the first activator of PMM2 that targets the root cause of disease.
INTRODUCTION
Deficiency of the enzyme phosphomannomutase 2 (PMM2) caused by loss-of-function mutations in the human PMM2 gene was shown over two decades ago to be the basis of a recessive congenital disorder of glycosylation originally called CDG1 or CDG1a. The first clinical observation by Jaeken and colleagues of a ‘carbohydrate-deficient glycoprotein syndrome’ occurred four decades ago (Jaeken et al., 1980). The researcher and patient communities now refer to the disease as PMM2-CDG, which is the most common congenital disorder of glycosylation and affects at least 1000 patients worldwide (Chang et al., 2018). Classical pediatric clinical presentations include developmental delay, severe encephalopathy with axial hypotonia, abnormal eye movements, psychomotor retardation and cerebellar hypoplasia (Matthijs et al., 1997). As patients reach their teenage years and young adulthood, health challenges include hypogonadism, coagulation abnormalities and thrombotic events, retinitis pigmentosa and peripheral neuropathy (Monin et al., 2014) The prognosis for PMM2-CDG patients is poor and there is currently no FDA-approved treatment that alleviates the symptoms of PMM2-CDG or any targeted therapy that safely increases PMM2 enzyme activity.
The PMM2 enzyme forms an obligate homodimer in the cytoplasm that converts mannose-6-phosphate to mannose-1-phosphate, an initial essential step in the N-linked glycosylation of proteins. Glucose-1,6-bisphosphate and mannose-1,6-bisphosphate are endogenous coactivators of PMM2 function, binding to and stabilizing PMM2 dimers (Andreotti et al., 2015). N-linked protein glycosylation is an evolutionarily conserved process that occurs in all animal cells throughout development and adulthood (Chang et al., 2018). PMM2-CDG is a multisystem, multi-organ disease because a minimal level of glycosylation is required at all times in all cells of the body, with different cell types and organs more or less vulnerable to the complex sequelae of hypoglycosylation. Although a clear genotype–phenotype relationship is obscured by genetic and possibly environmental modifiers, as the residual level of PMM2 enzymatic activity increases, the number and severity of organ systems affected decreases. For example, patients homozygous for a mutation in the promoter of PMM2 do not get PMM2-CDG or even a mild form of PMM2-CDG, but instead have hyperinsulinemic hypoglycemia and polycystic kidney disease because this mutation impairs binding by a kidney- and pancreas-specific transcription factor to a chromatin loop in the promoter of PMM2 (Cabezas et al., 2017). As another example, hypoglycosylation of the calcium channel CACNA1A causes a gain-of-function channelopathy that in turn leads to an increase in stroke-like events in PMM2-CDG patients (Izquierdo-Serra et al., 2018).
Complete loss of N-linked protein glycosylation uniformly results in lethality of all animals in which PMM2 has been genetically knocked out, including humans. Homozygotes of the most common pathogenic variant, R141H, which is catalytically null, have never been observed alive in spite of the statistical predictions of population genetics (Matthijs et al., 1998; Kjaergaard et al., 1998). Those results indicate that there is a lower bound of PMM2 enzymatic activity (3-7%) required for viability. However, the minimum PMM2 enzymatic activity above which disease is suppressed is unknown. Human genetics proves that this safety threshold varies from tissue to tissue and across stages of development. It further suggests that there are sharp tissue-specific transitions from physiology to pathophysiology, with buffering capacity determined by both common and rare genetic modifiers in N-linked glycosylation and related metabolic pathways (Citro et al., 2018).
Over 80% of disease-causing PMM2 alleles are missense mutations resulting in amino acid substitutions that destabilize an otherwise catalytically competent protein. Missense mutations fall into at least three biochemical classes: (1) protein destabilizing or misfolding mutations randomly distributed throughout the protein, (2) dimerization-defective mutations located in the monomer–monomer interface, and (3) ‘catalytically dead’ mutations in the active site (Yuste-Checa et al., 2015). Each PMM2 monomer forms a dimer with itself as a prerequisite for catalytic activity, although there need only be one functional active site per dimer (Andreotti et al., 2015). Many PMM2-CDG patients across ethnic populations share the compound heterozygous genotype R141H/F119L, which pairs the aforementioned R141H null allele with the F119L dimerization-defective allele. Typically, patients present with R141H in compound heterozygosity with a hypomorphic missense allele, often rare or private variants. Citro and colleagues argue that the unusual tolerance of PMM2 to missense mutations compared with every other CDG gene suggests a fitness advantage to being a PMM2 heterozygous carrier (Citro et al., 2018). As PMM2 enzymatic activity dips below 50%, disease symptoms arise; how severely they arise is unknown because of the contribution of genetic modifiers that buffer the safety thresholds between health and disease. Similarly, it is unknown how small a boost in PMM2 enzymatic activity above disease baseline is required to elicit a therapeutic effect in PMM2-CDG patients.
Challenges for the PMM2-CDG community have been inviable mouse models and the lack of a gold standard cellular model of disease, given the fact that N-linked protein glycosylation is a process that occurs in all cell types throughout life. A morpholino-knockdown model of PMM2-CDG in zebrafish displayed what appeared to be disease-relevant phenotypes, but these results have not been confirmed in a genetic knockout mutant (Cline et al., 2012). Fly models of PMM2-CDG were generated and also exhibited what appeared to be disease-relevant phenotypes but they suffered from early larval lethality, which is a difficult phenotype to rescue in a genetic or chemical screen (Parkinson et al., 2016). For those reasons, Perlara PBC developed the first yeast models of PMM2-CDG (Lao et al., 2019).
Previously, we established evolutionarily conserved genotype–phenotype relationships across yeast and human patients between five PMM2 disease-causing mutations and their orthologous mutations in yeast. Overexpression of PMM2 in yeast cells lacking SEC53 (the yeast ortholog of PMM2) rescued lethality by mass action effects and showed that there was no toxicity associated with overexpression of either PMM2 or SEC53 in yeast (Lao et al., 2019). Modest increases in SEC53 enzymatic activity translated into large phenotypic gains in yeast cell fitness. If the transition from physiology to pathophysiology is steep, is the transition from pathophysiology back to physiology equally steep? Are these sharp transitions conserved from yeast to humans? If the slopes of those transitions are conserved, small molecules are an attractive therapeutic modality not only because activation of PMM2 enzymatic activity appears to be well tolerated but also because modest boosts in PMM2 enzymatic activity might be sufficient to produce real-world clinical benefit and disease modification.
An academic drug discovery effort for PMM2-CDG involved expression of recombinant human PMM2 protein in bacteria, a primary target-based differential scanning fluorimetry screen and a secondary fibroblast-based PMM2 enzymatic activity assay in order to identify pharmacological chaperones (Yuste-Checa et al., 2017). Although this approach proved the concept that it is possible to discover pharmacological chaperones and novel chemotypes that increase PMM2 enzymatic activity, only one early-stage tool compound was identified and it is far from clinical candidacy. Another approach proposed by Andreotti and colleagues is to increase the levels of glucose-1,6-bisphosphate (G16BP), an endogenous stabilizer and coactivator of PMM2 dimers (Monticelli et al., 2019). They propose that G16BP could be used as a therapeutic pharmacological chaperone if it could be safely and effectively delivered to cells in vivo. We reasoned that rather than supplying exogenous chemically modified G16BP there may be small molecule drugs that pharmacologically increase endogenous G16BP by activating its synthesis or blocking its degradation, or both. The biotech company Glycomine is developing an oral liposomal mannose-1-phosphate substrate replacement therapy that is currently in Phase 1.
In order to identify drug repurposing candidates that boost PMM2 enzyme function, we generated and characterized the first worm patient avatar of PMM2-CDG as an intermediate translational model situated between our previously published yeast models (Lao et al., 2019) and well-established PMM2-CDG patient fibroblasts. We created a viable hypomorphic loss-of-function F119L homozygous mutant worm for use in a primary in vivo phenotypic drug screen and a secondary in vitro worm PMM2 enzyme activity assay. We showed that this F119L hypomorphic mutant exhibits hypersensitivity to bortezomib, a proteasome inhibitor and endoplasmic reticulum (ER) stress inducer, resulting in early larval arrest. Compounds that rescue arrested larvae and allow for normal development could activate ER stress response pathways to overcome chronic proteome-wide hypoglycosylation, boost PMM2 enzyme activity or boost PMM2 protein abundance. We adapted a low-throughput PMM2 enzymatic activity assay to a multiwell plate assay using PMM2-CDG patient fibroblasts (Van Schaftingen and Jaeken, 1995) in order to determine which clinical candidates from model organism drug repurposing screens can increase human PMM2 enzymatic activity.
The majority of repurposing candidates are ‘generally recognized as safe’ plant-derived polyphenols or phytochemicals, specifically a structurally related group of antidiabetic and antioxidant dietary flavonoids. These flavonoids (e.g. fisetin, ellagic acid) appear to share a common target and mechanism of action with a cinnamic acid derivative that can specifically rescue a F119L yeast model of PMM2-CDG (Lao et al., 2019), namely aldose reductase inhibition. Based on structure–activity relationships, we found that epalrestat, a generic diabetic peripheral neuropathy drug and the only safe aldose reductase inhibitor (ARI) approved for use in humans (Hotta et al., 2006), is a first-in-class PMM2 enzyme activator. The efficacy of epalrestat in the four genotypically distinct PMM2-CDG fibroblasts tested in this study suggests that epalrestat could be given to PMM2-CDG patients who are compound heterozygous for R141H and any pathogenic variant. However, epalrestat treatment of PMM2-CDG fibroblasts did not increase PMM2 protein levels as measured by immunoblotting, suggesting that aldose reductase inhibition acts post-translationally to boost PMM2 enzyme activity. We propose that aldose reductase inhibition leads to an increase in glucose-1,6-bisphosphate levels as a result of glucose being shunted away from the polyol pathway toward production of phosphorylated glucose. Evidence from epalrestat-treated pmm-2 mutant worms suggests there may also be a minor role for NRF2 as an indirect transcriptional activator of PMM2, which is consistent with a role for NRF2 activation in rescuing hypoglycosylation stress phenotypes in pmm2 mutant zebrafish (Mukaigasa et al., 2018).
Epalrestat is an oral formulation of an ARI that has been commercially approved and available in Japan since 1992 for the treatment of diabetic neuropathies. It is also commercially available in India and China. The drug was never commercially approved in the USA or EU but it has a long and safe history. The drug's ability to improve safely the symptoms of neuropathy alone by reducing oxidative stress, increasing glutathione levels and reducing intracellular sorbitol accumulation make it a desirable medication for PMM2-CDG patients who commonly suffer with various neuropathies. Based on the data presented herein, the newly discovered PMM2 enzyme activation mechanism of epalrestat may reduce the severity of the morbidities associated with PMM2-CDG.
RESULTS
Generation and phenotyping of the first nematode model of PMM2-CDG
We addressed the lack of animal models for PMM2-CDG amenable to unbiased high-throughput phenotypic screens by creating a series of worm patient avatars. The F52B11.6 gene sequence in Caenorhabditis elegans is orthologous to human PMM2. Nematode PMM-2 shares 54% identity with the human protein, and the two most common disease-causing mutations (R141H and F119L) are evolutionarily conserved. We first characterized a heterozygous pmm-2 null strain. This strain has a 518 base-pair deletion in the pmm-2 open reading frame. This deletion strain is documented to be larval lethal, which we confirmed (data not shown). The heterozygous animals produce few homozygous null progeny, insufficient for the scale of a high-throughput drug screen. We used quantitative RT-PCR to measure the amount of worm pmm-2 mRNA expression in pmm-2 heterozygote null animals. We observed that worm pmm-2 mRNA levels are the same in wild-type N2 animals and pmm-2 heterozygote null animals, indicating that this mutant compensates for hemizygosity at the transcriptional level by boosting PMM-2 expression to levels normally expressed in wild-type worms with two functional copies of pmm-2 (Fig. S1). Because pmm-2 homozygous nulls are inviable and pmm-2 heterozygous nulls do not exhibit overt growth and developmental delay phenotypes, we engineered a novel pmm-2 hypomorphic mutant strain using CRISPR/Cas9 and examined it for phenotypes amenable to a high-throughout image-based growth assay and a low-throughput whole-animal PMM-2 enzyme activity assay.
We created a homozygous F125L/F125L missense mutant, as worm F125 is orthologous to human F119. F119 sits in the dimer interface and the F119L mutation results in a defect in dimerization. The homozygous pmm-2 F125L (hereafter referred to as F119L) strain is homozygous viable and does not exhibit larval lethality, growth defects or any observable locomotor defects in liquid media. We verified that worm pmm-2 mRNA transcript levels in the F119L mutant are comparable with those in wild-type N2 animals (Fig. S1). To determine whether the F119L homozygote mutant is a bona fide model of PMM2-CDG, we measured worm PMM-2 enzyme activity in F119L homozygotes after whole-animal lysis. As expected, this mutant had reduced PMM-2 enzymatic activity; by contrast, PMM-2 enzymatic activity was unchanged in a png-1 homozygous null mutant and model of NGLY1 deficiency (another glycosylation disorder) and in wild-type N2 worms (Fig. 1).
Fig. 1.
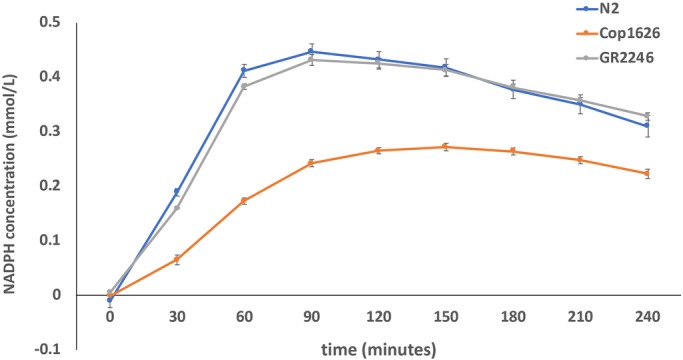
Plot of PMM2 enzymatic activity in whole-animal lysates of pmm-2F126L mutant worms. PMM2 activity is measured by production of NADPH concentration (mmol/L) over time. The blue line indicates PMM2 activity in wild-type N2 worms. The orange line indicates PMM2 activity in COP1626 worms (pmm-2F125L homozygote mutant). The gray line indicates PMM2 activity in GR2246 worms, a png-1 deletion mutant, which serves as a negative control. The same amount of protein was used in lysates from each strain.
Drug repurposing screen using pmm-2 F125L/F125L mutant worms
Because the F119L homozygous mutant strain presented no constitutive growth and developmental timing differences compared with wild-type worms, it was not amenable to high-throughput screening as is. We tested whether pmm-2 mutant animals were more sensitive to pharmacological stressors that disrupt proteasomal processes and induce ER stress. Literature evidence shows that tunicamycin (Buzzi et al., 2011) and bortezomib (Tillman et al., 2018) exposure causes proteasomal stress, activates the unfolded protein response and induces larval arrest in worms. We treated F119L mutant worms with increasing concentrations of tunicamycin and bortezomib. We sought to establish whether either of the compounds affected the growth and development of mutant animals at concentrations lower than in similarly exposed wild-type worms. Tunicamycin did not produce differential larval arrest in mutants and wild-type worms, possibly because the F119L mutation is not sensitive enough (data not shown). It is possible that tunicamycin might produce a differential larval arrest in the context of modeling a more severe PMM2 variant in worms.
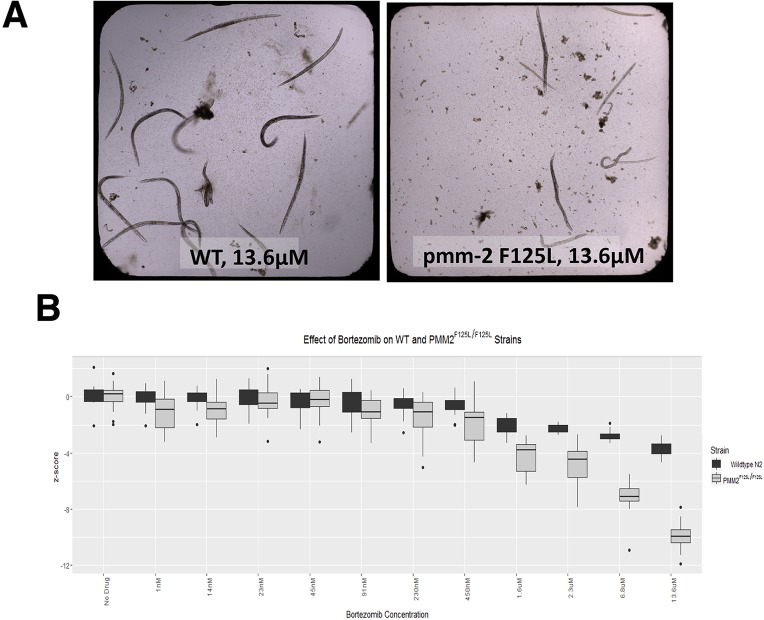
As shown in Fig. 2, we found that pmm-2 mutant worms exposed to bortezomib, a reversible proteasome inhibitor, displayed larval arrest at concentrations more than tenfold lower than in the wild type. At the highest concentration tested (13.6 µM), F119L mutant worms uniformly arrested as small larvae with morphological defects or were dead, whereas wild-type worms at this dose were viable late-stage larvae or young adults (Fig. 2A). Starting at 1.6 µM bortezomib, the size distribution of pmm-2 mutant worms separated from the size distribution of wild-type worms. At concentrations higher than 6.8 µM, pmm-2 mutant worms arrested as small sick larvae with no overlap between mutant and wild-type worm size distributions (Fig. 2B). We decided to screen the Microsource Spectrum drug repurposing library in triplicate for compounds that rescue early larval arrest in the presence of 11 μM bortezomib, a concentration that guaranteed robust statistical separation of positive control Z-scores (DMSO-treated pmm-2 mutant worms) from negative control Z-scores (bortezomib-treated pmm-2 mutant worms). We had previously screened the Microsource Spectrum library using yeast models of PMM2-CDG (Lao et al., 2019). We anticipated identifying overlapping chemotypes active in both species.
Fig. 2.
pmm-2 F125L homozygote mutant worms are more than ten times more sensitive to bortezomib than wild-type worms. (A) Side-by-side comparison of representative wells of clear-bottom 384-well plates containing wild-type worms (left) and pmm-2 F125L/F125L worms (right) treated with 13.6 µM bortezomib, the highest dose tested. (B) Z-score box and whisker plot comparing pmm-2 F125L/F125L worms (gray boxes) and wild-type worms (black boxes) treated with the following range of concentrations of bortezomib: 1 nM, 14 nM, 23 nM, 45 nM, 91 nM, 230 nM, 450 nM, 1.6 µM, 2.3 µM, 6.8 µM, 13.6 µM. Z-score labels on the y-axis are 0, −4, −8 and −12. Individual black dots represent statistical outliers.
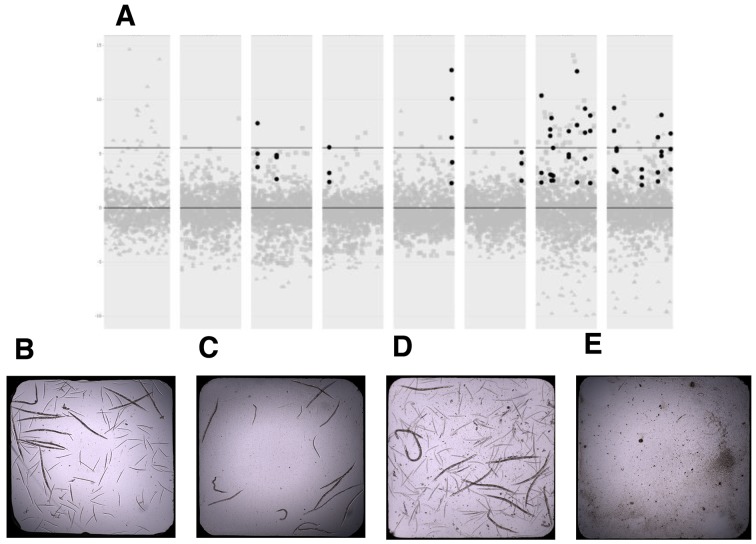
Representative positive control wells and negative control wells are shown in Fig. 3. Note that the worms in negative control wells were still viable even if they were arrested (Fig. 3B). Statistically significant separation between positive and negative controls allowed us to identify both suppressors and enhancers, which included toxic compounds (Fig. S2). We only examined suppressors in the context of this study (i.e. compounds that fully rescued early larval arrest induced by 11 µM bortezomib). Note that the worms in wells containing suppressors looked indistinguishable from worms in positive control wells (Fig. 3D versus Fig. 3B). Twenty compounds had Z-scores greater than five in all three replicates (Fig. 3A), resulting in a suppressor hit rate of 0.781%, which was consistent with the hit rates we observed in previous whole-organism phenotypic drug repurposing screens in flies (Rodriguez et al., 2018) and yeast (Lao et al., 2019).
Fig. 3.
Summary of drug repurposing screen of pmm-2 F125L/F125L mutant worms. (A) Three replicates of the Microsource Spectrum library screen (replicate 1, gray circles; replicate 2, gray squares; replicate 3, gray triangles). Black circles represent the 20 hit compounds with Z-scores greater than five in all three replicates. Each column represents one of the eight library plates. The lower black line indicates Z-score of 0. The upper black line indicates Z-score of 5. (B) Image of a representative positive control well into which L1 DMSO-treated pmm-2 F125L/F125L mutant worms were dispensed and incubated at 20°C for 5 days. 12 adults are visible along with their progeny. (C) Representative negative control well into which L1 11 µM bortezomib-treated pmm-2 F125L/F125L mutant worms were dispensed and incubated at 20°C for 5 days. 16 animals are visible, ranging from L1-L4 larval stages. (D) Representative suppressor (hit) well into which L1 pmm-2 F125L/F125L mutant worms were dispensed and incubated at 20°C for 5 days. 12 adults are visible along with their progeny. (E) Representative enhancer/toxic well into which L1 11 µM-bortezomib-treated pmm-2 F125L/F125L mutant worms were dispensed and incubated at 20°C for 5 days. No living larvae are visible.
Repurposing candidates are antidiabetic and antioxidant phytochemicals
Of the 20 repurposing candidates (suppressors), 12 (60%) are generally recognized as safe (GRAS) dietary polyphenols found in fruits, vegetables, roots, spices and trees – specifically aglycone flavonoids and flavonoid glycosides: fisetin, rhamnetin, pyrogallin, purpurogallin-4-carboxylic acid, quercetin tetramethyl ether, ellagic acid, gossypetin, hieracin (tricetin), baicalein, koparin, epicatechin monogallate and theaflavin monogallate. Three (15%) of the hit compounds (3-methoxycatechol, 2,3,4-trihydroxy-4-methoxybenzophenone and 3,4-didesmethyl-5-deshydroxy-3-ethoxyschleroin) are the simple building blocks or scaffolds of more complex polyphenols, and for that reason were not considered further. Another three (15%) are catecholamines (levodopa, ethylnorepinephrine and dobutamine). Levodopa is a precursor to dopamine. Ethylnorepinephrine is a sympathomimetic drug and bronchiodilator. Dobutamine is a β1-adrenergic receptor agonist approved for the treatment of heart failure. The remaining two chemically simple compounds (amidol and edavarone) may be classified as nonspecific antioxidants.
Plant-based polyphenols have complex polypharmacology, meaning they are biologically active at multiple protein and nonprotein targets in the cell. As a result, plant-based polyphenols have multiple mechanisms of action depending on the concentration tested and length of treatment in a diverse array of in vitro and in vivo models. What unites many dietary phytochemicals are their antioxidant and anti-inflammatory properties, which in human physiology translates to known antidiabetic (hypoglycemic) and potential senolytic effects.
Pyrogallin contains a tropolone moiety and is the decarboxylated derivative of purpurogallin-4-carboxylic acid. Fisetin, gossypetin, hieracin, baicalein and koparin are all structurally related flavones differing in the number and location of hydroxyl groups. Quercertin tetramethyl ether and rhamnetin are O-methylated derivatives of quercetin, the most consumed dietary flavonoid. Ellagic acid results from the hydrolysis of more complicated tannins. Epicatechin monogallate and theaflavin monogallate, which contains the tropolone core scaffold pyrogallin, are flavanols present in green and black teas. It is known that gossypetin can directly inactivate bortezomib, resulting in false positive hits, so we removed it from further consideration (Glynn et al., 2015).
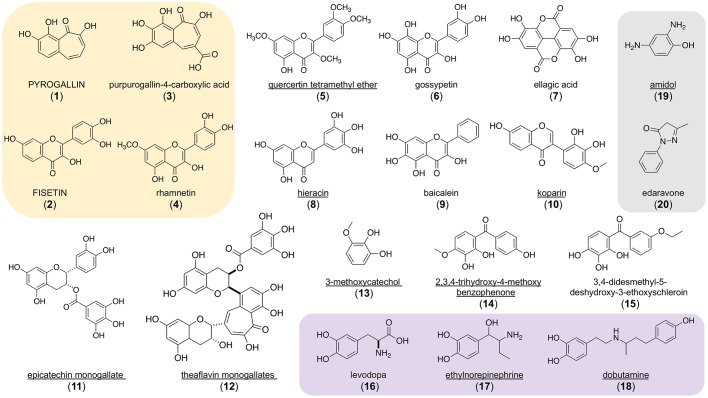
The chemical structures of all 20 PMM2 repurposing candidates are shown in Fig. 4. Remarkably, our flavonoid repurposing candidates have all been reported to be ARIs in the single to low double digit micromolar concentration range, and therefore would display this activity under the conditions of the primary drug repurposing screen and secondary hit validation assays. Fisetin, quercetin tetramethylether and rhamnetin were shown to inhibit aldose reductase, with half-maximal inhibitory concentrations of 3.7, 25 and 2.7 µM, respectively (Matsuda et al., 2002). Quercetin was identified as an ARI in 1975 (Varma et al., 1975, 1977).
Fig. 4.
Chemical structures of 20 drug repurposing candidates discovered in a worm pmm-2 bortezomib chemical modifier screen. Fully capitalized compounds are active in the human PMM2 enzyme activity assay performed in R141H/F119L PMM2-CDG fibroblasts as described in Materials and Methods. Underlined compounds are active in at least one of three yeast PMM2-CDG models described by Lao et al. (2019). The yellow box indicates compounds that are active in the Keap1-Nrf2 cellular reporter assay described in Materials and Methods. The purple box indicates compounds that are catecholamines. The grey box indicates structural singletons with nonselective antioxidant properties. (1) pyrogallin, (2) fiseti,. (3) purpurogallin-4-carboxylic acid, (4) rhamnetin, (5) quercetin tetramethyl ether, (6) gossypetin, (7) ellagic acid, (8) hieracin (tricetin), (9) baicalein, (10) koparin, (11) epicatechin monogallate, (12) theaflavin monogallate, (13) 3-methoxycatechol, (14) 2,3,4-trihydroxy-4-methoxybenzophenone, (15) 3,4-didesmethyl-5-deshydroxy-3-ethoxyschleroin, (16) levodopa, (17) ethylnorepinephrine, (18) dobutamine, (19) amidol, (20) edaravone.
The flavonoid 2′-2′-bisepigallocatechin digallate (Fig. S3) was identified as one of three repurposing candidates in our previously published yeast PMM2 drug repurposing study (Lao et al., 2019) and is structurally related to theaflavin digallate. This result demonstrated that specific plant-based polyphenols rescue growth by a conserved mechanism of action in both yeast and worm species paradigms. Plant-based polyphenols also are all well-known antioxidants that activate cytoprotective responses such as the Keap1-Nrf2 pathway, which is not conserved in yeast. We tested the 20 worm repurposing candidates in yeast PMM2-CDG models and in a human cell-based Keap1-Nrf2 activity assay.
Cross-species retesting of worm repurposing candidates in yeast and fibroblasts
We performed two secondary screens in order to prioritize the 20 worm repurposing candidates for final validation testing in PMM2-CDG patient fibroblasts in a human PMM2 enzyme activity assay representing the most common genotype R141H/F119L. First, we tested all 20 worm repurposing candidates in three genotypically distinct yeast PMM2-CDG models (Lao et al., 2019). Ten (50%) of the worm repurposing candidates improved growth of one or more yeast PMM2 models (Fig. 4; Fig. S7). Theaflavin monogallate, epicatechin monogallate and koparin and ethylnorepinephrine rescued two out of three yeast PMM2-CDG models but were not considered further. Quercetin tetramethylether, amidol and dobutamine rescued growth in a dose-dependent manner in all three yeast PMM2-CDG models. Quercetin tetramethylether had the greatest effect, so we began to suspect that it acts as an ARI because aldose reductase is conserved in yeast.
It is possible that inactivity in the yeast PMM2-CDG models could be the result of compound insolubility or instability in yeast media versus in worm media, or other organism-specific assay variables. To dissect the polypharmacology of plant-based polyphenols, we tested all 20 worm repurposing candidates in a Keap1-Nrf2 reporter human cell assay. We reasoned that plant-based polyphenols are known antioxidants, and some have been reported to activate the transcriptional regulator NRF2. Compounds that scored positively in the Keap1-Nrf2 reporter assay were then tested in a PMM2 enzyme activity assay on PMM2-CDG R141H/F119L fibroblasts in order to triage the list of compounds down to the few most promising repurposing candidates. We optimized PMM2 enzymatic assays for worms and patient fibroblasts based on the previously reported protocol (Van Schaftingen and Jaeken, 1995).
When we tested all 20 worm repurposing hits in a Keap1-Nrf2 reporter assay in human cells, NRF2 was activated by only 4/20 (20%) of the compounds: fisetin, rhamnetin, pyrogallin and purpurogallin-4-carboxylic acid (Fig. 4). Fisetin is a known NRF2 activator (Smirnova et al., 2011). The other three were not previously known to activate NRF2. None of the four worm repurposing candidates that activate the Keap-Nrf2 reporter assay rescued any of the yeast PMM2-CDG models, which is the expected result given the lack of conservation of NRF2 in yeast. Pyrogallin, purpurogallin-4-carboxylic acid, rhamnetin and fisetin did not rescue any of the yeast PMM2-CDG models tested. In fact, all four inhibited the growth of all three yeast PMM2-CDG yeast models tested, with pyrogallin and purpurogallin-4-carboxylic acid having a larger effect than fisetin and rhamnetin (Fig. S6).
Validating aldose reductase inhibitors in PMM2 enzymatic assays in worms and fibroblasts
Next, we tested the four worm repurposing candidates that activated the Keap1-Nrf2 pathway in a human PMM2 enzymatic activity assay using a R141H/F119L PMM2-CDG patient fibroblast line. We also tested the three yeast PMM2 repurposing candidates reported by Lao et al. (2019) whose structures are shown in Fig. S3. Of those seven compounds, only the yeast repurposing candidate α-cyano-4-hydroxycinnamic acid (CHCA) robustly activated PMM2 enzymatic activity, not only in PMM2-CDG R141H/F119L fibroblasts but also in the F119L mutant worm (Fig. 5; chemical structure shown in Fig. S3). CHCA (15 µM) increased worm PMM2 enzymatic activity by 50% over baseline but the variance was so large that the result was not statistically significant (P=0.4175). Similarly, 10 µM CHCA increased human PMM2 enzymatic activity by 40%, which was marginally statistically significant because of the variance (P=0.0436), as shown in Fig. 5B. The worm repurposing candidates pyrogallin, purpurogallin-4-carboxylic acid and fisetin activated human PMM2 enzymatic activity in PMM2-CDG R141H/F119L fibroblasts (Fig. 4), but inhibited all three yeast PMM2-CDG models at all three concentrations tested. Therefore, we deprioritized those three compounds and focused on CHCA.
Fig. 5.
Aldose reductase inhibitors CHCA and epalrestat increase PMM2 enzymatic activity in worms and patient fibroblasts. (A) Percentage of PMM2 enzymatic activity normalized to the pmm-2 F125L/F125L (F119L) homozygote mutant treated with DMSO vehicle. From left to right: pmm-2 F125L/F125L (F119L) homozygote mutant treated with DMSO (grey), 15 µM epalrestat for 24 h (P=0.0352) (yellow) or 15 µM CHCA for 24 h (P=0.4175) (orange). (B) Percentage of PMM2 enzymatic activity relative to wild type (100%). 50% represents the activity level of unaffected heterozygous carriers. From left to right: wild-type control fibroblasts treated with DMSO vehicle (blue), GM20942 R141H/F119L PMM2-CDG patient fibroblasts treated with DMSO vehicle (grey), 10 µM epalrestat (P=0.00825) (yellow) or 10 µM CHCA (P=0.0436) (orange). The same amount of protein was used in lysates from each strain. Error bars represent standard error. P-values were determined by unpaired t-test (T.TEST function in Excel).
CHCA is a potent ARI (Zhang et al., 2016), which is consistent with the known ARI mechanism of action of dietary flavonoids. The predominant ARI pharmacophore contains a terminal carboxylic acid moiety, as does CHCA (Fig. S8). We reasoned that the variance associated with CHCA might be caused by its relative pharmacological promiscuity and off-target effects. Therefore, we tested the following nine commercially available ARIs representing the known ARI pharmacophores, including those containing the terminal carboxylic acid moiety shared with CHCA: tolrestat, ranirestat, imirestat, zopolrestat, sorbinil, ponalrestat, alrestatin, fiderastat and epalrestat. Tolrestat, imirestat, ponalrestat and alrestatin increased PMM2 enzymatic activity in only one of three replicates (data not shown).
Of the ARIs tested, only carboxylic acid-containing epalrestat and CHCA reproducibly increased PMM2 enzymatic activity in both worms (Fig. 5A) and fibroblasts (Fig. 5B; Fig. S4). In pmm-2 F119L mutant worms, 15 µM epalrestat treatment caused a 30% increase in PMM2 enzymatic activity (P=0.0352). In R141H/F119L PMM2-CDG fibroblasts, 10 µM epalrestat also caused a 30% increase in PMM2 enzymatic activity over baseline (P=0.00825). Epalrestat is the only ARI that is orally bioavailable, brain-penetrant, well tolerated and approved for use in humans. Epalrestat was approved in Japan in 1992 for the treatment of diabetic neuropathy in geriatric patients (Hotta et al., 1996); it is also available in China and India, but has not been approved by the Food and Drug Administration (FDA) for any indications. In light of these data, we deprioritized CHCA, which is not approved for use in humans, in favor of the safe and generic epalrestat that has been used for decades in Asia.
Epalrestat boosts PMM2 enzyme activity in multiple PMM2-CDG patient fibroblasts
We tested whether the ARI epalrestat increases human PMM2 enzymatic activity in more than one PMM2 genotype and genetic background. Even though the compound heterozygous genotype R141H/F119L is the most common genotype observed in PMM2-CDG patients worldwide, there is a long tail of private missense mutations in trans with R141H so we tested three additional genotypes: R141H/E139K, R141H/N216I and R141H/F183S. Incubation of human fibroblasts derived from PMM2-CDG patients with 10 µM epalrestat for 24 h led to an increase in PMM2 enzymatic activity in all tested samples (Fig. 6). As summarized in Table 1, each PMM2-CDG fibroblast line had a different PMM2 enzymatic activity baseline, reflecting the severity of the respective PMM2 mutations correlating with enzyme activity impairment.
Fig. 6.
Epalrestat boosts human PMM2 enzymatic activity in multiple PMM2-CDG patient fibroblasts. (A) Supplemented samples were treated with 10 µM epalrestat for 24 h. PMM2 enzymatic activity was measured in PMM2-CDG fibroblasts with the genotype R141H/E139K (blue circles), in PMM2-CDG fibroblasts with the genotype R141H/N216I (red squares) and in PMM2-CDG fibroblasts with the genotype R141H/F183S (green triangles). (B) Bar plot of mean PMM2 enzymatic activity with or without epalrestat treatment (P=0.0321). Each black circle indicates the mean of replicates for each fibroblast. Error bars in the bar graphs indicate standard error of means. P-value was determined by t-test (T.TEST function in Excel).
Table 1.
Assay of PMM2 enzymatic activity in PMM2-CDG patient fibroblasts
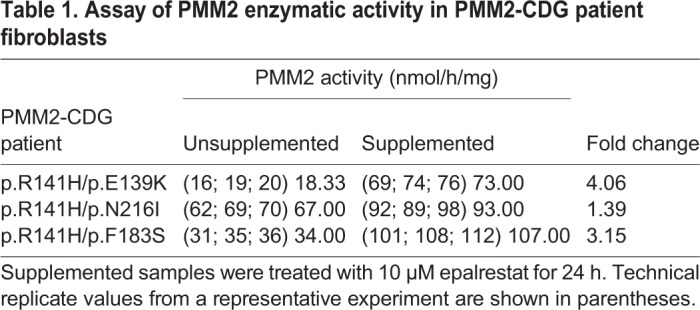
Patient R141H/E139K fibroblasts showed the lowest unsupplemented PMM2 enzyme activity but attained the largest relative increase in PMM2 enzyme activity after 24 h of epalrestat treatment: 4.06-fold increase from 18 to 73 nmol/h/mg. Patient R141H/N216I fibroblasts had the highest PMM2 enzyme activity baseline and attained the smallest relative increase in PMM2 enzyme activity with epalrestat: 1.39-fold increase from 67 to 93 nmol/h/mg. Patient R141H/F183S fibroblasts showed robust rescue by epalrestat: 3.15-fold increase from 34 to 107 nmol/h/mg. Averaging across the three genotypes, PMM2 activity increased 2.29-fold over the vehicle-treated baseline (P=0.0321). Supplemented PMM2 activity did not reach the formal patient control reference activity value of >700 nmol/h/mg, yet the identification of a several-fold increase in PMM2 activity in response to 10 µM epalrestat in 24 h represents a proof-of-concept for the therapeutic application of epalrestat in remediation of loss of PMM2 enzymatic activity.
Epalrestat does not increase PMM2 protein abundance
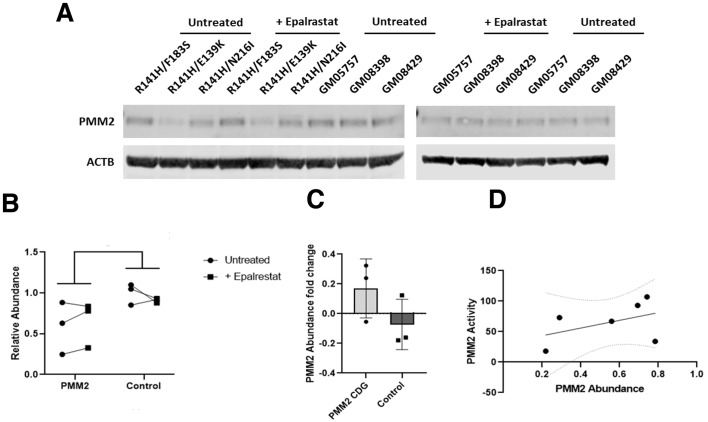
The simplest model to explain how epalrestat increases PMM2 enzyme activity is that epalrestat increases PMM2 protein abundance. We demonstrated previously that PMM2 overexpression rescues lethality of yeast models of PMM2-CDG by mass action effects (Lao et al., 2019). Immunoblotting with antibody specific to PMM2 clearly showed that epalrestat treatment did not increase PMM2 protein abundance in PMM2-CDG fibroblasts or in control fibroblasts (Fig. 7A).
Fig. 7.
Epalrestat treatment does not increase PMM2 protein abundance. (A) Immunoblots showing PMM2 protein levels in epalrestat-treated PMM2-CDG patient fibroblasts and control fibroblasts. Actin was used a loading control. (B) Quantification of PMM2 protein abundance based on band intensity. Black circles represent 10 µM epalrestat-treated PMM2-CDG fibroblasts. Black squares represent untreated control fibroblasts. (C) PMM2 protein abundance fold change before and after 10 µM epalrestat treatment for 24 h. Black circles represent epalrestat-treated PMM2-CDG fibroblasts. Black squares represent untreated control fibroblasts. (D) Plot of PMM2 enzymatic activity as a function of PMM2 protein abundance. Black circles represent three PMM2-CDG patient fibroblast lines and three control fibroblasts.
In two out of three PMM2-CDG fibroblasts, the PMM2 protein abundance increased in response to epalrestat treatment. However, in the third PMM2-CDG fibroblast, PMM2 protein abundance decreased upon epalrestat treatment and we observed the reverse trend in three control fibroblasts (Fig. 7B). We averaged PMM2 protein abundance fold change in epalrestat-treated PMM2-CDG fibroblasts and compared it to the averaged PMM2 protein abundance fold change in control fibroblasts (Fig. 7C). There was no statistically significant difference. Furthermore, we observed a weak positive trend between baseline PMM2 protein abundance versus baseline PMM2 enzyme activity (Fig. 7D). Together, these results demonstrate that the elevated PMM2 enzyme activity observed in fibroblasts treated with epalrestat for 24 h can be explained by greater catalytic efficiency of PMM2 protein already present in the cell rather than by greater PMM2 protein abundance (i.e. simple mass action).
Epalrestat increases pmm-2 mRNA expression and rescues ER stress markers in worms
It was recently reported that a pmm2 hypomorphic mutant zebrafish constitutively activates NRF2 and furthermore that the small molecule NRF2 activator sulforaphane ameliorates ER-associated stress markers (Mukaigasa et al., 2018). Therefore, we tested whether epalrestat acts similarly to sulforaphane by interrogating mRNA expression levels of worm orthologs of the ER stress markers that are induced in a pmm2 hypomorphic mutant zebrafish. The results of this quantitative PCR analysis are shown in Fig. S5. Worm pmm-2 transcript levels were increased by 15 µM epalrestat. Transcript levels of hsp-1, which encodes a heat shock protein, were also increased by epalrestat treatment. Conversely, the ER stress markers ire-1, pek-1, skn-1 and gcs-1, which are constitutively elevated in the F119L mutant, were modestly decreased by treatment with epalrestat. These results bolster confidence in the translational fidelity of our F119L worm model of PMM2-CDG because it phenocopies the constitutively active ER stress response seen in a zebrafish model of PMM2-CDG.
DISCUSSION
In this study, we set out to build on the results of our previous yeast PMM2-CDG disease modeling and drug repurposing efforts (Lao et al., 2019). We have filled a gap in PMM2-CDG disease models by generating a nematode model based on a specific PMM2-CDG variant, in this case the second most common missense allele F119L (F125L in worms). We confirmed that the F119L nematode model has 30-40% residual PMM-2 enzymatic activity, comparable to a haploid F119L yeast model. We identified hypersensitivity to the proteasome inhibitor bortezomib as a growth-based phenotype in an unbiased whole-organism drug repurposing screen. A comparative analysis of yeast and worm PMM2 repurposing hits revealed overlap in one structural class: plant-based polyphenols. Using a PMM2 enzymatic activity assay in PMM2-CDG patient fibroblasts, we showed that several yeast and worm repurposing candidates can increase PMM2 enzymatic activity. These compounds are the first known small molecule activators of PMM2 enzymatic activity.
Analysis of structure–activity relationships and cross-species testing suggested that plant-based polyphenols act as ARIs. Testing of commercially available ARIs showed conclusively that epalrestat boosts PMM2 enzymatic activity in both nematodes and PMM2-CDG patient fibroblasts. Epalrestat is the only ARI approved for the treatment of diabetic neuropathy in humans and has been used safely for nearly three decades. It has been used to treat peripheral neuropathy in geriatric diabetic patients in Asia for several decades. We believe our results justify repurposing epalrestat for PMM2-CDG, starting with a small safety and efficacy study involving up to ten PMM2-CDG patients. The only other example of drug repurposing for PMM2-CDG involves the carbonic anhydrase inhibitor acetazolamide; the therapeutic thesis is that acetazolamide will treat the cerebellar syndrome of PMM2-CDG but not correct the root cause of disease, which is PMM2 enzyme insufficiency (Martínez-Monseny et al., 2019).
What is the mechanism of action by which epalrestat potentiates PMM2 enzymatic activity? We initially hypothesized that activation of NRF2 would lead to increased PMM2 mRNA levels, which in turn would lead to increased PMM2 protein abundance. Consistent with that hypothesis, treatment of pmm2 zebrafish mutant with the NRF2 activator sulforaphane resulted in rescue of phenotypic defects (Mukaigasa et al., 2018). We know from our studies of yeast PMM2-CDG models that overexpression of F119L mutant PMM2 protein can rescue growth by mass action effects (Lao et al., 2019). Four plant-based polyphenols (fisetin, rhamnetin, pyrogallin and purpurogallin-4-carboxylic acid) were active in the Keap1-Nrf2 cellular reporter assay, but only fisetin and pyrogallin boosted PMM2 enzymatic activity. Several reports indicate that epalrestat activates NRF2 in cultured cells (Yama et al., 2016, 2015). Using quantitative RT-PCR, we showed that epalrestat treatment of pmm-2 F125L/F125L homozygote worms modestly upregulated skn-1 transcripts (skn-1 is the worm ortholog of NRF2). As a direct test of this model, we quantified PMM2 protein abundance in PMM2-CDG patient fibroblasts by immunoblotting. Epalrestat did not increase PMM2 protein abundance in any of the fibroblast lines tested.
Therefore, we favor a model whereby epalrestat acts post-translationally to boost PMM2 enzymatic activity by preventing glucose from being shunted down the polyol pathway. Aldose reductase is the first biosynthetic enzyme in the polyol pathway, and ARIs block the conversion of glucose to sorbitol. Excess glucose would then favor the production of glucose-1,6-bisphosphate, an endogenous small molecule coactivator of PMM2 (Pirard et al., 1999; Monticelli et al., 2019). At the same time, inhibition of the polyol pathway reduces the levels of advanced glycation end products, which would attenuate the sequelae of ER stress and oxidative stress. In humans, epalrestat is known to reduce the levels of carboxymethyl lysine, an advanced glycation end product (Kawai et al., 2010). It is also possible that there is a complex interaction between activation of NRF2 and inhibition of the polyol pathway, the former acting at the transcriptional level and the latter acting post-translationally.
Our model makes several testable predictions: First, the concentration of glucose-1,6-bisphosphate will increase in yeast, worms and human cells treated with epalrestat. Second, the concentration of sorbitol will decrease in yeast, worms and human cells treated with epalrestat. Third, CRISPR knockout of aldose reductase in PMM2-CDG patient fibroblasts will block activation of PMM2 enzyme activity by epalrestat. Meanwhile, decades of safe and effective administration of epalrestat for peripheral neuropathy in geriatric populations suggests that the drug may also be safe in the pediatric settings necessitated by PMM2-CDG. Peripheral neuropathy is observed in almost all PMM2-CDG patients, so a therapeutic rationale for repurposing epalrestat for PMM2-CDG is compelling.
There are over 180 publications on the use of epalrestat in the peer-reviewed literature of the last 25 years. Epalrestat has been studied in three pivotal registration studies, and multiple post-market studies have been conducted. The drug has an excellent safety profile, with the most common side effects reported being nausea, vomiting, diarrhea and elevated liver enzymes; the most severe side effect reported is liver failure.
Will the PMM2 enzyme activity increase in epalrestat-treated patient fibroblasts lead to clinical benefit in patients? The expectation is that epalrestat can be used to treat the peripheral neuropathy of PMM2-CDG patients, but will it have impact beyond peripheral neuropathy? Based on the Mayo Clinic PMM2-CDG biobank, 55 affected fibroblast lines exhibit a range of PMM2 enzymatic activity between 18 and 307 nmol/h/mg. Mayo Clinic uses a diagnostic cutoff at 307 nmol/h/mg. A total of 66 unrelated noncarrier control fibroblast lines exhibit a range of PMM2 enzymatic activity between 712 and 2066 nmol/h/mg. Mayo Clinic reports healthy PMM2 enzymatic activity as any value >712 nmol/h/mg. Clinicians working on PMM2-CDG believe that >100 nmol/h/mg of PMM2 enzymatic activity would translate to clinical benefit.
MATERIALS AND METHODS
Strains, cell lines and compounds
The pmm-2 mutant VC3054 was obtained from the Caenorhabditis Genetics Center (CGC). It is a homozygous lethal deletion chromosome balanced by GFP-marked translocation. COP1626 is the pmm-2 F125L (F119L in humans) hypomorphic homozygous mutant that was generated using CRISPR/Cas9 by NemaMetrix (Eugene, OR). The R141H/F119L patient fibroblast line GM20942, the R141H/E139K patient fibroblast line GM27386, the control fibroblast GM05757, the control fibroblast GM08398 and the control fibroblast GM08429 were obtained from Coriell (Camden, NJ). The R141H/N216I patient fibroblast line and the R141H/F183S patient fibroblast line were obtained from the Mayo Clinic biobank. Screening was conducted using the 2560-compound Microsource Spectrum library consisting of FDA-approved drugs, bioactive tool compounds and natural products. For all retests, compounds were reordered from Spectrum Discovery as 5 mg dry powder stocks. Compounds were solubilized with fresh dimethylsulfoxide (DMSO) at high concentrations of 100 mM or 50 mM and stored as aliquots at −20°C. For worm retesting, a 10 mM stock was prepared.
High-throughput larval growth assays in worms
The F125L/F125L homozygous pmm-2 mutant growth screen was conducted in 384-well plates. Wells were filled with 25 μM of compound (137.5 nl) from the Microsource Spectrum library, dispensed into 320 wells of the plate using an Echo 550 acoustic dispenser from Labcyte (San Jose, CA). In addition, all test wells were dispensed with 12.5 nl of a 50 mM stock of bortezomib solubilized in DMSO, resulting in a final concentration of 11 μM. Control wells of the plate were filled with 150 nl of DMSO (positive control) or 12.5 nl of bortezomib and 137.5 nl of DMSO (negative control). Following addition of test compounds, wells were dispensed with 5 μl of bacterial medium resuspended in S-medium containing cholesterol after adjusting the optical density to 0.45 at 600 nm. Mutant animals were grown on standard nematode growth medium agar plates until gravid. Adult worms were bleached using hypochlorite treatment to obtain eggs. Eggs were allowed to hatch overnight at 20°C in order to obtain synchronized L1 larvae. L1 larvae were filtered through 15 micron filters to ensure that the resulting population was clean before sorting. Larvae were then dispensed (15 per well) into the 384-well plate using a BioSorter large particle flow cytometer from Union Biometrica. Plates were sealed and incubated on a shaker at 20°C for 5 days. On the fifth day, plates were vortexed and spun down before addition of 15 μl of 8 mM sodium azide. The addition of sodium azide immobilized worms, after which they were imaged under transmitted light using a custom plate imager. Automated image processing was run on each plate.
Data analysis and statistical methods for the growth assay in worm
We used the statistical program R to calculate Z-scores after processing the raw data, and we used Excel to calculate P-values using t-tests. We used custom image processing algorithms to extract areas occupied by worms per well. After outlier elimination among controls (i.e. elimination of data points because of image or experimental artifacts), Z-scores were assigned to each well of the plate relative to the negative controls. Next, all wells that had a Z-score of greater than two in triplicate were isolated and manually inspected. Because bortezomib suppresses larval growth and induces arrest, we determined that a compound rescued the underlying defect if a well had a larger area occupied by worms relative to the negative control. Visual inspection often revealed that in primary screening positive wells, worms attained adulthood and produced progeny.
PMM2 enzymatic assay in worms
L1-stage-synchronized F125L/F125L homozygous pmm-2 mutant animals were grown (2000 worms per plate) on NGM agar for 24 h. After 24 h, worms were washed off plates into 15 ml conical tubes, washed with filtered autoclaved water, pelleted at 3200 rpm for 4 min and then resuspended in 2 ml S-medium. HB101 bacteria grown in LB overnight were pelleted at 4000 rpm for 10 min and resuspended in S-medium with cholesterol to an optical density of 0.35. For each experimental condition, 25 ml of HB101 in S-medium was added to 50 ml conical tubes along with 15,000-20,000 worms. Test compounds were dissolved in DMSO stock solutions and added to samples at a final concentration of 15 µM. Samples were incubated at 20°C for 24 h. After 24 h, conical tubes were placed on ice and worms were allowed to settle for 15 min. The bacterial supernatant was aspirated, and the worms were pelleted and washed with water. Worms were transferred to 1.5 ml Eppendorf tubes, pelleted at 4°C and lysed in 70-100 µl homogenization buffer (20 mM HEPES, 25 mM KCl, 1 mM DTT, 10 µg/ml leupeptin, 10 µg/ml antipain) on ice. The lysate was centrifuged, and 1-2 µl of lysate was used for protein quantification using a Qubit. Lysate equivalent of 10 µg protein was used per well to determine PMM-2 enzyme activity after adding the 200 µl of assay buffer. The assay was carried out for 3-4 h, with absorbance readings every 30 min at 340 nm. Assay buffer comprised 50 mM HEPES pH 7.1, 5 mM MgCl2, 0.25 mM NADP+, 10 µg/ml yeast glucose-6-phosphate dehydrogenase, 10 µM glucose-1,6-bisphosphate, 200 µM mannose-1-phosphate, 10 µg/ml phosphoglucoseisomerase and 3.5 µg/ml phosphomannoseisomerase. Assay buffer without the substrate mannose 1-phosphate was used as the control.
PMM2 enzymatic assay development in R141H/F119L patient fibroblasts
Briefly, cells were seeded in a 96-well plate, homogenization buffer (20 mM HEPES, 25 mM KCl, 1 mM DTT, 10 µg/ml leupeptin, 10 µg/ml antipain) was added and plates were freeze-thawed at −80°C twice to lyse cells. Reaction buffer (50 mM HEPES, 5 mM MgCl2, 0.5 mM NADP+, 10 µg/ml yeast glucose-6-phosphate dehydrogenase, 10 µM glucose-1,6-bisphosphate, 10 µg/ml phosphoglucoisomerase, 5.25 µg/ml phosphomannoseisomerase) containing 200 µM mannose-1-phosphate as substrate was then added to the wells of each plate.
Plates were incubated at 37°C for 270 min and absorbance was read at 340 nm at 30, 60, 90, 120, 150, 180, 210, 240 and 270 min by pulling the plate out of incubation at the respective time points. All incubations were carried out with or without substrate (mannose-1-phosphate) and the difference between the two values taken as the enzymatic activity. Enzyme activity was normalized to total lysate protein levels. Enzyme activities of wild-type fibroblasts and the patient-derived compound heterozygous (F119L/R141H) fibroblast line were determined.
To assess whether compounds from the phenotypic screens affected enzyme activity, all compounds were incubated with the mutant cell line at a concentration of 10 μM for a period of 24 h. Following this, enzyme activity was assessed as described above. At least two biological replicates were conducted and enzyme activity in the presence of a test compound was compared with that of a DMSO-treated mutant cell line. For ease of analysis, we compared the enzyme activity (as represented by NADPH concentration) of each treatment condition with the activity of a baseline, untreated mutant line at the last time point.
Epalrestat treatments in optimized PMM2 enzymatic activity assay
Fibroblast samples from PMM2-CDG patients R141H/F119L, GM20942 and GM27386 and from control fibroblasts GM05757, GM08398 and GM08429 were cultured in the presence and absence of 10 µM epalrestat for 24 h. Samples were lysed using sonication, after which total protein concentration was determined using the BioRad LSR total protein detection assay. For each sample, 1.0 mg/ml protein was used for further analysis. After lysis, samples were centrifuged at 10,000 rpm for 10 min. After centrifugation, 80 µl of supernatant was transferred to a microtiter plate. PMM2 substrate mannose-1-phosphate was added to each sample, after which the plate was transferred to a FLUOstar Omega Plate Reader to determine fluorescent excitation after 30 and 40 min as a measure of PMM2 enzyme activity. All samples were run in duplicate, with each run including healthy control and disease control standards. Enzyme activity scores are presented as nanomoles per hour per milligram (nmol/h/mg) protein based on enzyme activity constants calculated using the following formula:
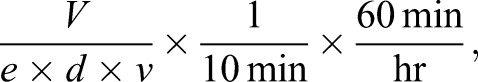 |
where total volume V=0.250 ml, extinction coefficient e=6.22×10−3 cm2/nmol (NADPH), path length d=0.828 cm and sample volume v=0.05 ml.
Keap1-NRF2 activation luciferase reporter assay in U2OS cells
Hit compounds were tested by DiscoverX (San Diego) using the PathHunter eXpress Keap1-NRF2 Nuclear Translocation Assay. Briefly, PathHunter cells were plated and incubated for 24 h at 37°C. Test compound (10 µl) was added and cells incubated with compound for 6 h at room temperature. Working detection reagent solution was added and plates were incubated for 60 min at room temperature. Chemiluminescence signal was read by a SpectraMax M3. Methyl CDDO ester was used as the positive control. To be called a NRF2 activator, a hit compound had a half-maximal effective concentration (EC50) less than 10 µM and a slope comparable to that obtained for methyl CDDO ester.
PMM2 protein quantification in fibroblasts by immunoblotting
To isolate protein, cells were washed in 1× PBS and pelleted. Cell pellets were solubilized in RIPA Buffer+Protein Inhibitor Cocktail. Protein concentration was determined using the BCA method. Immunoblotting was performed as follows: 30 µg cell protein was separated on a 10% Bis-Tris gel (NP0301PK2, Thermo Fisher Scientific) and blotted onto nitrocellulose membranes using the standard protocol provided by the manufacturer. Rabbit polyclonal antibody against human PMM2 (10666-1-AP, Proteintech) was diluted 1:333 (30 µl in 10 ml blocking buffer). Mouse monoclonal antibody against human ACTB (AC004, CiteAb) was diluted 1:15,000 (0.66 µl in 10 ml blocking buffer). Membranes were blocked for 30 min at 4°C in SEA Block blocking buffer (37527, Thermo Fisher Scientific). Primary antibodies were diluted in 10 ml SEA Block. Membranes were incubated on a rocker in primary antibody dilution overnight at 4°C. Membranes were washed six times for 10 min in 40 ml 1× PBS containing 0.1% Tween at room temperature. Donkey anti-mouse green (SA5-10172, Thermo Fisher Scientific) and donkey anti-rabbit red (SA5-10042, Thermo Fisher Scientific) fluorescent antibodies were diluted 1:5000 (2 µl each in 10 ml blocking buffer). Membranes were incubated in secondary antibody dilution for 1 h at 4°C. Membranes were then washed six times for 10 min in 40 ml 1× PBS containing 0.1% Tween at room temperature. Finally, membranes were washed once for 10 min in 40 ml 1× PBS at room temperature and immediately visualized.
Quantitative RT-PCR of worms
Worms were age-synchronized and collected as day 1 adults. Worm pellets were homogenized and RNA extracted following published protocols (Guthmueller et al., 2011). ER stress markers were selected based on the pmm2 zebrafish model (Mukaigasa et al., 2018).
Supplementary Material
Acknowledgements
We thank the anonymous reviewers for feedback that significantly improved the manuscript.
Footnotes
Competing interests
The following authors are shareholders of Perlara PBC: S.I., N.D., F.S.S., Z.P., K.M., H.T. and E.O.P.
Author contributions
Conceptualization: S.I., N.D., J.L., E.O.P.; Methodology: S.I., F.S.S., N.D., J.V., K.M., Z.P.; Software: H.T.; Validation: S.I., F.S.S., J.V., K.M., E.M.; Formal analysis: S.I., F.S.S., G.P., Z.P., H.T., J.L., E.M., E.O.P.; Investigation: S.I., G.P., Z.P., J.L.; Data curation: K.M., H.T.; Writing - original draft: E.O.P.; Writing - review & editing: S.I., J.V., J.L., E.M.; Visualization: F.S.S., J.V., K.M., E.M.; Supervision: S.I., N.D., G.P., J.L., E.M., E.O.P.; Project administration: S.I., N.D., J.L., E.M., E.O.P.; Funding acquisition: E.O.P.
Funding
We acknowledge Maggie's PMM2-CDG Cure, LLC as a primary funding source. This work was also supported by the National Institutes of Health [1 U54 NS115198-0 to E.M.].
Data availability
High-throughput screening datasets are publically available as ‘Perlara ArkBase v1’ on Collaborative Drug Discovery (https://www.collaborativedrug.com; free registration required at https://app.collaborativedrug.com/register).
Supplementary information
Supplementary information available online at http://dmm.biologists.org/lookup/doi/10.1242/dmm.040584.supplemental
References
- Andreotti G., Monti M. C., Citro V. and Cubellis M. V. (2015). Heterodimerization of two pathological mutants enhances the activity of human phosphomannomutase2. PLoS ONE 10, e0139882 10.1371/journal.pone.0139882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzi L. I., Simonetta S. H., Parodi A. J. and Castro O. A. (2011). The two Caenorhabditis elegans UDP-glucose:glycoprotein glucosyltransferase homologues have distinct biological functions. PLoS ONE 6, e27025 10.1371/journal.pone.0027025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas O. R., Flanagan S. E., Stanescu H., García-Martínez E., Caswell R., Lango-Allen H., Antón-Gamero M., Argente J., Bussell A.-M., Brandli A. et al. (2017). Polycystic kidney disease with hyperinsulinemic hypoglycemia caused by a promoter mutation in phosphomannomutase 2. J. Am. Soc. Nephrol. 28, 2529-2539. 10.1681/ASN.2016121312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang I. J., He M. and Lam C. T. (2018). Congenital disorders of glycosylation. Ann. Transl. Med. 6, 477 10.21037/atm.2018.10.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citro V., Cimmaruta C., Monticelli M., Riccio G., Hay Mele B., Cubellis M. V. and Andreotti G. (2018). The analysis of variants in the general population reveals that PMM2 is extremely tolerant to missense mutations and that diagnosis of PMM2-CDG can benefit from the identification of modifiers. Int. J. Mol. Sci. 19, 2218 10.3390/ijms19082218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline A., Gao N., Flanagan-Steet H., Sharma V., Rosa S., Sonon R., Azadi P., Sadler K. C., Freeze H. H., Lehrman M. A. et al. (2012). A zebrafish model of PMM2-CDG reveals altered neurogenesis and a substrate-accumulation mechanism for N-linked glycosylation deficiency. Mol. Biol. Cell 23, 4175-4187. 10.1091/mbc.e12-05-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn S. J., Gaffney K. J., Sainz M. A., Louie S. G. and Petasis N. A. (2015). Molecular characterization of the boron adducts of the proteasome inhibitor bortezomib with epigallocatechin-3-gallate and related polyphenols. Org. Biomol. Chem. 13, 3887-3899. 10.1039/C4OB02512A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmueller K. L., Yoder M. L. and Holgado A. M. (2011). Determining genetic expression profiles in C. elegans using microarray and real-time PCR. J. Vis. Exp. 53, e2777 10.3791/2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta N., Sakamoto N., Shigeta Y., Kikkawa R., Goto Y. and Diabetic Neuropathy Study Group in Japan (1996). Clinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. J. Diabet. Compl. 10, 1996 10.1016/1056-8727(96)00113-4 [DOI] [PubMed] [Google Scholar]
- Hotta N., Akanuma Y., Kawamori R., Matsuoka K., Oka Y., Shichiri M., Toyota T., Nakashima M., Yoshimura I., Sakamoto N. et al. (2006). Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care 29, 1538-1544. 10.2337/dc05-2370 [DOI] [PubMed] [Google Scholar]
- Izquierdo-Serra M., Martínez-Monseny A. F., López L., Carrillo-García J., Edo A., Ortigoza-Escobar J. D., García Ó., Cancho-Candela R., Carrasco-Marina M. L., Gutiérrez-Solana L. G. et al. (2018). Stroke-like episodes and cerebellar syndrome in phosphomannomutase deficiency (PMM2-CDG): evidence for hypoglycosylation-driven channelopathy. Int. J. Mol. Sci. 19, 619 10.3390/ijms19020619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J., Vanderschueren-Lodeweyckx M., Casaer P., Snoeck L., Corbeel L., Eggermont D. and Eeckels R. (1980). Familial psychomotor retardation with markedly fluctuating serum prolactin, FSH and GH levels, partial TBG-deficiency, increased serum arylsulphatase A and increased CSF protein: a new syndrome? Pediatr. Res. 14, 179 10.1203/00006450-198002000-00117 [DOI] [Google Scholar]
- Kawai T., Takei I., Tokui M., Funae O., Miyamoto K., Tabata M., Hirata T., Saruta T., Shimada A. and Itoh H. (2010). Effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy in patients with type 2 diabetes, in relation to suppression of N(ɛ)-carboxymethyl lysine. J. Diabet. Compl. 24, 424-432. 10.1016/j.jdiacomp.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Kjaergaard S., Skovby F. and Schwartz M. (1998). Absence of homozygosity for predominant mutations in PMM2 in Danish patients with carbohydrate-deficient glycoprotein syndrome type 1. Eur. J. Hum. Genet. 6, 331-336. 10.1038/sj.ejhg.5200194 [DOI] [PubMed] [Google Scholar]
- Lao J. P., DiPrimio N., Prangley M., Sam F. S., Mast J. D. and Perlstein E. O. (2019). Yeast models of phosphomannomutase 2 deficiency, a congenital disorder of glycosylation. G3 9, 413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Monseny A. F., Bolasell M., Callejón-Póo L., Cuadras D., Freniche V., Itzep D. C., Gassiot S., Arango P., Casas-Alba D., de la Morena E. et al. (2019). AZATAX: acetazolamide safety and efficacy in cerebellar syndrome in PMM2 congenital disorder of glycosylation (PMM2-CDG). Ann. Neurol. 85, 740-751. 10.1002/ana.25457 [DOI] [PubMed] [Google Scholar]
- Matsuda H., Morikawa T., Toguchida I. and Yoshikawa M. (2002). Structural requirements of flavonoids and related compounds for aldose reductase inhibitory activity. Chem. Pharm. Bull. 50, 788-795. 10.1248/cpb.50.788 [DOI] [PubMed] [Google Scholar]
- Matthijs G., Schollen E., Pardon E., Veiga-Da-Cunha M., Jaeken J., Cassiman J.-J. and Van Schaftingen E. (1997). Mutations in PMM2, a phosphomannomutase gene on chromosome 16p13 in carbohydrate-deficient glycoprotein type I syndrome (Jaeken syndrome). Nat. Genet. 16, 88-92. 10.1038/ng0597-88 [DOI] [PubMed] [Google Scholar]
- Matthijs G., Schollen E., Van Schaftingen E., Cassiman J.-J. and Jaeken J. (1998). Lack of homozygotes for the most frequent disease allele in carbohydrate-deficient glycoprotein syndrome type 1A. Am. J. Hum. Genet. 62, 542-550. 10.1086/301763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin M.-L., Mignot C., De Lonlay P., Héron B., Masurel A., Mathieu-Dramard M., Lenaerts C., Thauvin C., Gérard M., Roze E. et al. (2014). 29 French adult patients with PMM2-congenital disorder of glycosylation: outcome of the classical pediatric phenotype and depiction of a late-onset phenotype. Orphanet J. Rare Dis. 9, 207 10.1186/s13023-014-0207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli M., Liguori L., Allocca M., Andreotti G. and Cubellis M. V. (2019). β-Glucose-1,6-bisphosphate stabilizes pathological phophomannomutase2 mutants in vitro and represents a lead compound to develop pharmacological chaperones for the most common disorder of glycosylation, PMM2-CDG. Int. J. Mol. Sci. 20, E4164 10.3390/ijms20174164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaigasa K., Tsujita T., Nguyen V. T., Li L., Yagi H., Fuse Y., Nakajima-Takagi Y., Kato K., Yamamoto M. and Kobayashi M. (2018). Nrf2 activation attenuates genetic endoplasmic reticulum stress induced by a mutation in the phosphomannomutase 2 gene in zebrafish. Proc. Natl. Acad. Sci. USA 115, 2758-2763. 10.1073/pnas.1714056115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson W. M., Dookwah M., Dear M. L., Gatto C. L., Aoki K., Tiemeyer M. and Broadie K. (2016). Synaptic roles for phosphomannomutase type 2 in a new Drosophila congenital disorder of glycosylation disease model. Dis. Model. Mech. 9, 513-527. 10.1242/dmm.022939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirard M., Achouri Y., Collet J.-F., Schollen E., Matthijs G. and Van Schaftingen E. (1999). Kinetic properties and tissular distribution of mammalian phosphomannomutase isozymes. Biochem. J. 339, 201-207. 10.1042/bj3390201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez T. P., Mast J. D., Hartl T., Lee T., Sand P. and Perlstein E. O. (2018). Defects in the neuroendocrine axis contribute to global development delay in a Drosophila model of NGLY1 deficiency. G3 8, 2193-2204. 10.1534/g3.118.300578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova N. A., Haskew-Layton R. E., Basso M., Hushpulian D. M., Payappilly J. B., Speer R. E., Ahn Y.-H., Rakhman I., Cole P. A., Pinto J. T. et al. (2011). Development of Neh2-luciferase reporter and its application for high throughput screening and real-time monitoring of Nrf2 activators. Chem. Biol. 18, 752-765. 10.1016/j.chembiol.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman E. J., Richardson C. E., Cattie D. J., Reddy K. C., Lehrbach N. J., Droste R., Ruvkun G. and Kim D. H. (2018). Endoplasmic reticulum homeostasis is modulated by the forkhead transcription factor FKH-9 during infection of Caenorhabditis elegans. Genetics 210, 1329-1337. 10.1534/genetics.118.301450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E. and Jaeken J. (1995). Phosphomannomutase deficiency is a cause of carbohydrate-deficient glycoprotein syndrome type I. FEBS Lett. 377, 318-320. 10.1016/0014-5793(95)01357-1 [DOI] [PubMed] [Google Scholar]
- Varma S. D., Mizuno A. and Kinoshita J. H. (1975). Flavonoids as inhibitors of lens aldose reductase. Science 188, 1215-1216. 10.1126/science.1145193 [DOI] [PubMed] [Google Scholar]
- Varma S. D., Mizuno A. and Kinoshita J. H. (1977). Diabetic cataracts and flavonoids. Science 195, 205-206. 10.1126/science.401544 [DOI] [PubMed] [Google Scholar]
- Yama K., Sato K., Abe N., Murao Y., Tatsunami R. and Tampo Y. (2015). Epalrestat increases glutathione, thioredoxin, and heme oxygenase-1 by stimulating Nrf2 pathway in endothelial cells. Redox Biol. 4, 87-96. 10.1016/j.redox.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yama K., Sato K., Murao Y., Tatsunami R. and Tampo Y. (2016). Epalrestat upregulates heme oxygenase-1, superoxide dismutase, and catalase in cells of the nervous system. Biol. Pharm. Bull. 39, 1523-1530. 10.1248/bpb.b16-00332 [DOI] [PubMed] [Google Scholar]
- Yuste-Checa P., Gámez A., Brasil S., Desviat L. R., Ugarte M., Pérez-Cerdá C. and Pérez B. (2015). The effects of PMM2-CDG-causing mutations on the folding, activity, and stability of the PMM2 protein. Hum. Mutat. 36, 851-860. 10.1002/humu.22817 [DOI] [PubMed] [Google Scholar]
- Yuste-Checa P., Brasil S., Gámez A., Underhaug J., Desviat L. R., Ugarte M., Pérez-Cerdá C., Martinez A. and Pérez B. (2017). Pharmacological chaperoning: a potential treatment for PMM2-CDG. Hum. Mutat. 38, 160-168. 10.1002/humu.23138 [DOI] [PubMed] [Google Scholar]
- Zhang L., Li Y.-F., Yuan S., Zhang S., Zheng H., Liu J., Sun P., Gu Y., Kurihara H., He R.-R. et al. (2016). Bioactivity focus of α-Cyano-4-hydroxycinnamic acid (CHCA) leads to effective multifunctional aldose reductase inhibitors. Sci. Rep. 6, 24942 10.1038/srep24942 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.