Abstract
Purpose
Rheumatoid arthritis (RA) can result from complex interactions between the affected person’s genetic background and environment. Viral and bacterial infections may play a pathogenetic role in RA through different mechanisms of action. We aimed to evaluate the presence of antibodies (Abs) directed against two proteins of Mycobacterium avium subsp. paratuberculosis (MAP) in sera of RA subjects, which are crucial for the survival of the pathogen within macrophages. Moreover, we analyzed the correlation of immune response to both proteins with the following homologous peptides: BOLF1305–320, MAP_402718–32 and IRF5424–434 to understand how the synergic role of Epstein–Barr virus (EBV) and MAP infection in genetically predisposed subjects may lead to a possible deregulation of interferon regulatory factor 5 (IRF5).
Materials and methods
The presence of Abs against protein tyrosine phosphatase A (PtpA) and protein kinase G (PknG) in sera from Sardinian RA patients (n=84) and healthy volunteers (HCs, n=79) was tested by indirect ELISA.
Results
RA sera showed a remarkably high frequency of reactivity against PtpA in comparison to HCs (48.8% vs 7.6%; p<0.001) and lower but statistically significant responses towards PknG (27.4% vs 10.1%; p=0.0054). We found a significant linear correlation between the number of swollen joints and the concentrations of antibodies against PtpA (p=0.018). Furthermore, a significant bivariate correlation between PtpA and MAP MAP_402718–32 peptide has been found, suggesting that MAP infection may induce a secondary immune response through cross-reaction with IRF5 (R2=0.5).
Conclusion
PtpA and PknG are strongly recognized in RA which supports the hypothesis that MAP infection may be involved in the pathogenesis of RA.
Keywords: Mycobacterium avium subsp. paratuberculosis, PtpA, PknG, virulence factors, rheumatoid arthritis, immune response
Introduction
Mycobacterium avium subsp. paratuberculosis (MAP) is an obligate intracellular pathogen. Humans are frequently exposed to MAP due to its prevalence in dairy cattle and because of environmental contamination and resistance to pasteurization and chlorination. This bacterium is the etiologic agent of Johne’s disease in ruminants and it has been associated with Crohn’s disease1–3 as well as some other human diseases.4 Following the colonization of the host, the parasitic organism alters the host immune system by the use of molecular mimicry, displaying peptide sequences similar to that of the host cells, causing a disruption of self vs non-self-recognition. Theoretically, the failure to recognize the invading organism from host cells may result in numerous autoimmune conditions. To demonstrate this, different studies conducted in recent decades show that MAP infection is dangerous to the animals, but the threat is felt most significantly by humans.5,6 It has been necessary to develop different laboratory techniques to better investigate the MAP infection. Interestingly, ELISA results showed a high sensitivity and specificity in comparison to the other tests.7,8
In this scenario, in addition to Crohn’s disease, MAP has been associated with other human diseases, like multiple sclerosis (MS)9–11 and neuromyelitis optica spectrum disorder (NMOSD).12,13 MAP has also been associated with Parkinson’s disease14,15 and with type-1 diabetes (T1D).16,17 In addition, it has been shown that in mice, heat-killed MAP (MIFA) emulsified in incomplete Freund’s (ICF) adjuvant, induced an early experimental autoimmune encephalomyelitis and more severe clinical scores, as opposed to MOG-CFA immunized mice.18 Recent studies suggest that MAP infection may trigger rheumatoid arthritis disease (RA),19,20 a common chronic joint inflammatory disease with aberrant synovial inflammation, proliferation of the synovial tissues, and advanced destruction of cartilage and bone.21–23 The etiology of RA is unknown but involves both environmental and genetic factors. It has been suggested that molecular mimicry between bacterial and human antigens may be one of the many possible mechanisms of RA development.20 Moreover, individuals with RA have autoantibodies to citrullinated peptides frequently, which suggests the involvement of the peptidylarginine deiminases citrullinating enzymes (encoded by PADIgenes) in RA.24–27
Recent studies highlight the crucial role of macrophages in RA.28,29 Considering the results following the stimulation of macrophages with LPS30 and taking into consideration that MAP infection specifically targets macrophages,49 we hypothesize that this may lead to a dysregulation of the molecular pathways. This may lead to a different expression of transcription factors and cytokines, causing a dysregulation of the inflammatory response in RA.
MAP infection, the cause of great losses of livestock, is now correlated with several autoimmune human diseases, thus proving a problem for public health.20,31 In a previous study, we identified a high sequence homology among interferon regulatory factor 5 (IRF5), EBV antigen BOLF1, and MAP antigen MAP_4027, and found that epitopes of EBV and MAP cross-react with IRF5.19 We have also demonstrated that IRF5 is a potential autoimmune target of RA.20 These results support the hypothesis that MAP infection might be involved in RA pathogenesis, igniting a secondary immune response that cross-react against RA self-peptides.19,20 To this end, we aim to further investigate the molecular mechanism involved during the MAP infection.
In this study, we analyzed the immune response against two proteins of MAP: PtpA (protein tyrosine phosphatase A; UniProtKB Accession number: A0A200GPK8) and PknG (protein kinase G; UniProtKB Accession number: A0A202FS53) in RA patients and HCs. Results showed that a high level of antibodies against both virulence factors was measured, suggesting that these patients had been previously exposed or infected with MAP.
Materials and Methods
Subjects
Eighty-four RA patients (19 males, 65 females; median age 59.0 years) who met the 2010 ACR/EULAR Classification Criteria for RA (criteria of the American College of Rheumatology)32 were enrolled at the Outpatient Clinic of the Rheumatology Unit, Department of Clinical and Experimental Medicine, University Hospital of Sassari, Italy. Collected data included: disease duration, rheumatoid factor (RF) positivity, anti-cyclic citrullinated peptide (anti-CCP) positivity, steroid treatment, disease-modifying anti-rheumatic drugs (DMARDs), anti-tumor necrosis factor-alpha drugs (tocilizumab, and abatacept), levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) levels, Disease Activity Score-28 (DAS-28), and Health Assessment Questionnaire (HAQ).
A total of 79 healthy controls (HCs) (25 males, 54 females; median age 46.7 years) were recruited at the Blood Transfusion Centre of Sassari, Italy. Demographic, clinical and laboratory features of RA patients and HCs are summarized in Table 1.
Table 1.
Demographic, Clinical and Laboratory Features of RA Subjects and HCs
| RA n=84 | HCs n=79 | |
|---|---|---|
| Age, years | 59.0 ± 9.9 | 46.7 ± 11.6 |
| Female sex, n (%) | 65 (77.38) | 54 (68.35) |
| Disease duration, months | 145 ± 134 | – |
| ACPA positivity, n (%) | 68.6 | – |
| RF positivity, n (%) | 74.5 | – |
| HAQ (0–3) | 0.92 ± 0.73 | – |
| DAS-28 | 3.72 ± 1.38 | – |
| CRP, mg/dL | 0.88 ± 0.96 | – |
| ESR, mm/h | 30.7 ± 25.8 | – |
| Steroids use, n (%) | 43.4 | – |
| DMARDs use, n (%) | 66.3 | – |
| Anti-TNF use, n (%) | 24.1 | – |
| Tocilizumab use, n (%) | 12 | – |
| Abatacept use, n (%) | 4.9 | – |
Recombinant Protein Production and Plate Preparation
Recombinant proteins were produced according to published protocols.33–36 In brief, PtpA was produced in M. smegmatis harboring the ptpA gene cloned into the vector pALACE (hygromycin resistance), whereas PknG was produced in E. coli harbouring the plasmid pknG-pET-30b (kanamycin resistance). Both proteins were purified by affinity chromatography using Ni-NTA resin as published.33 Produced proteins were stored at −20°C until used.
ELISA plates were Maxisorp (TermoFisher) and were coated with 50 μg/mL of each antigen in PBS overnight at 4°C. The next day, plates were washed with PBS, supplemented with Tween-20 (PBS-T) ×3 and blocked with BSA 3% in PBS overnight at 4°C. The concentration of antigen used in this study was already determined in previous studies as the concentration necessary to obtain a differential change in the readout.34–36 The next day, the blocking solution was discarded, and the plates were dried at room temperature. Plates were then shipped to Italy to perform the ELISA. Previous studies performed in our laboratory indicated that the shipping of dried plates did not affect the antigen conformation.35
ELISA Assays and Statistical Analysis
The study investigated specific antibody responses against PtpA and PknG proteins in RA patients in comparison to healthy controls using enzyme-linked immunosorbent assay (ELISA). The technique was performed as described previously.19 The optical density (OD) was read at a wavelength of 405 nm using SpectraMax Plus 384 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
All experiments were repeated three or more times and all serum samples were assayed in duplicates. The background, which was determined by incubating immobilized protein with a secondary antibody alone, was subtracted from each measurement. Positive control sera were included in all experiments. The cut off for positivity in each assay was calculated by ROC analysis and set at >90% specificity. The sensitivity was chosen accordingly. The significance of differences between the OD values of RA and HCs groups was determined using a Mann–Whitney test.
Linear regression analysis adjusted for age and sex was performed to evaluate the correlation between RA features (independent variables) and the serum concentration of antibodies against PtpA and PknG (dependent variables).
Differences with p<0.05 were considered statistically significant. The area under receiver operating characteristic curve (AUROC) was analyzed using GraphPad Prism 6.0 software (San Diego, CA, USA).
Comparison of positivity to the assessed proteins between RA patients and HCs was performed through Fisher’s exact test.
Correlation analyses were performed between PtpA and PknG and all the homologous pairs (BOLF1, MAP_4027 and IRF5).
The study was approved by the Ethics Committee of the Azienda Ospedaliero-Universitaria of Cagliari, Italy (prot. Num. PG/2018/5463) and performed in accordance with the Declaration of Helsinki. All participants or their legal tutors have given written informed consent. All methods were carried out in accordance with the approved guidelines.
Results
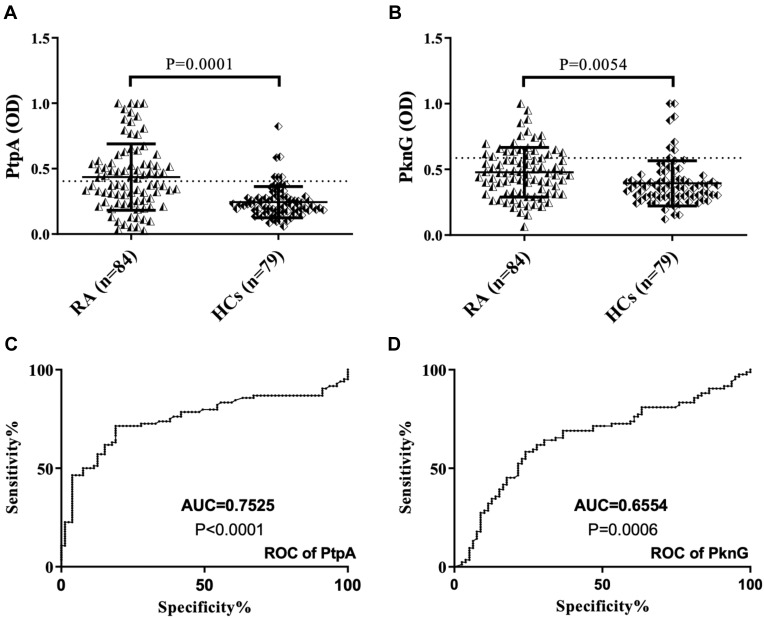
Abs against PtpA were found to be above positive level in 41 out of 84 (48.8%) RA patients, whereas 6 out of 79 (7.6%) HCs were positive in serum (AUC=0.7525, p=0.0001, Figure 1A–C). Regarding PknG, 23 out of 84 (27.4%) RA patients, 8 out of 79 (10.12%) HCs were positive in serum (AUC=0.6554, p=0.0054, Figure 1B–D).
Figure 1.
ELISA-based analysis of antibody reactivity against two proteins of MAP in RA subject and HCs. The sera was tested against plate-coated PtpA (A) and PknG (B) proteins. Bars represent the median ± interquartile range. Thresholds for antibody positivity are indicated by dashed lines. P-values are indicated above the distributions. ROC analysis of both proteins (C and D).
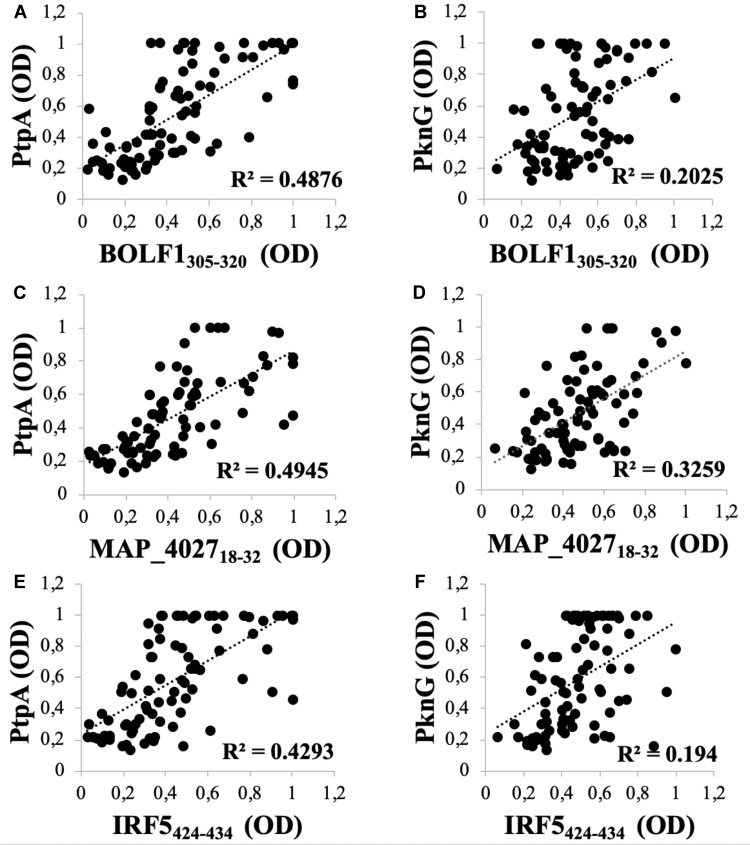
A noteworthy correlation was found between PtpA and all homologous pairs (BOLF1, MAP_4027 and IRF5). The highest correlation was found between PtpA and MAP_402718–32 (R2=0.4945; Figure 2C) followed by BOLF1305–320 (R2=0.4876; Figure 2A). IRF5424–434 results showed the lowest correlation with PtpA, compared to MAP_4027 and BOLF1305–320, but it is still well founded to hypothesize a cross-reactivity between the two epitopes (R2=0.4293; Figure 2E). On the other hand, we found lower correlations between PknG and all three homologous pairs, in comparison with PtpA, i.e., (R2=0.2025; Figure 2B) with BOLF1305–320, (R2=0.194; Figure 2F) with IRF5424–434 and (R2=0.3259; Figure 2D) with MAP_402718-32. The correlation analysis showed that EBV and MAP have a synergic effect in the pathogenesis of RA.
Figure 2.
Scatter plot showing correlations between Abs titers recognizing (A) BOLF1305–320 and PtpA, (C) MAP_402718–32 and PtpA, (E) IRF5424–434 and PtpA, (B) BOLF1305–320 and PknG, (D) MAP_402718–32 and PknG, (F) IRF5424–434 and PknG in 84 RA patients and 79 HCs. Person’s correlation was calculated through Graphpad Prism 6.0 software.
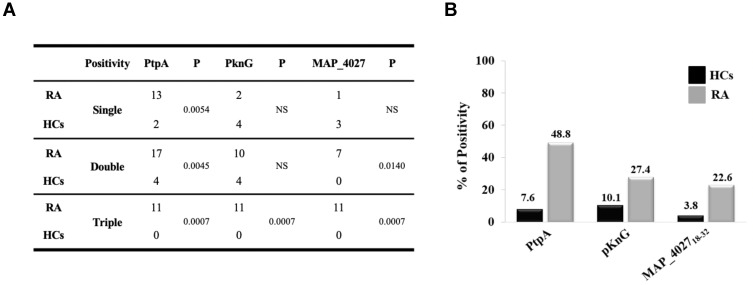
Globally, RA subjects displayed a significantly increased positivity to PtpA protein compared to HCs with a high degree of statistical significance (Figures 1A and 3A). However, we have found a lower reactivity with PknG (Figures 1B and 3A). Finally, a lower reactivity of MAP_402718–32 was observed when comparing to PtpA and PknG, but with a statistical significance vs HCs (22.6% vs 3.8%, p=0.0004, Figure 3B).
Figure 3.
(A) Coincidence of seroreactivity to the PtpA, PknG proteins and MAP_402718–32 peptide among Abs-positive RA and HCs subject. (B) Prevalence of Abs directed against PtpA, PknG and MAP_402718–32 in RA patients and HCs.
We found no correlation between anti-PtpA/PknG Abs and serological and clinical features of RA, including disease activity and the type of immunosuppressive treatment, with the exception of a marginal correlation between Abs against PtpA serum concentration and a number of tender and swollen joints (r=0.224, p=0.042 and r=0.279, p=0.010, respectively). In the linear regression analysis, performed adjusting for age and sex, a significant linear correlation between the number of swollen joints and the serum concentration of Abs against PtpA was found (B (95% CI) = 0.025 (0.004–0.045), p=0.018).
Analyzing single, double, and triple positivity for PtpA, PknG and MAP_402718–32 peptide, we observed that 11 RA patients tested positive for all antigens, while no positivity was found with HCs (p=0.0007, Figure 3A). These data showed that in an attempt to delete the pathogen, the immune system triggers an immune reaction against different portions of MAP but with a major reactivity for PtpA.
The results showed that, generally, PtpA and PknG proteins are highly recognized in RA patients, which may be useful to analyze the molecular mechanisms in macrophages after MAP infection. It is thus helpful to understand how MAP grows inside the macrophage to understand its implication in RA pathogenesis.
Discussion
The pathogenesis of RA is due to a complicated interplay between genes, the immune system, and the environment.37 In this study, we investigated further the link between MAP and macrophages in RA, taking into consideration that different cell populations are involved in the autoimmune process. However, it became increasingly clear that macrophages are very important in the pathogenesis of RA, as they generate cytokines that enhance inflammation and contribute to the destruction of bone and cartilage. Research has now discovered an unexpectedly high level of heterogeneity in the macrophages’ origin and function, and has also emphasized the role of environmental factors in their functional specialization.28 Bacterial and viral infections and other environmental factors can modify the function of macrophages by modulating the expression of transcription factors. MAP is an intracellular bacterium that should grow in macrophages, and for this reason, we suspect that this infection can lead to a dysregulation of these cells.
We analyzed the immune response against PtpA and PknG, which are crucial proteins necessary for the survival of the pathogen within macrophages, in RA patients and HCs. In addition, we evaluated the existence of a correlation among immune responses against MAP_4027, BOLF1, and IRF5. This is helpful for understanding how the synergic role of EBV and MAP infection in genetically predisposed subjects can lead to a possible deregulation of IRF5.
We found that the Abs level against both MAP proteins is significantly higher in RA patient sera in comparison with HC sera. Moreover, we suspect that these patients have been either exposed or infected with MAP, which supports our theory on the impact of MAP infection on the development of RA.
Our results are in line with corresponding studies that showed that sera of sheep previously infected with MAP, as well as in Crohn’s disease and MS have significantly higher levels of reactivity to PtpA.1–3,12 Similarly, we have found that in RA patients there is a strong immune response compared to HCs, and hence PtpA is a candidate for the potential detection of humoral immune responses in human.
Among different environmental agents that have been associated with RA in recent studies, there is some evidence that associates MAP with RA.20 Recent studies have also suggested that MAP is involved in the immune response of several autoimmune diseases such as Crohn’s disease, MS, NMOSD and T1D, as previously indicated.1,2,9–12,16,17 The proposed mechanism is based on molecular mimicry and citrullination.38 In addition to the bacterium Porphyromonas gingivalis that has been associated with RA both in the murine model39 and in humans,26 other pathogens can also be involved, either independently or with a synergistic effect.40,41 In fact, in a work under review, we highlight the presence of a statistically significant antibody response between RA and HCs against the citrullinated peptide of MAP_402718–32Cit (date not shown). Using Fisher’s exact test we compared MAP_4027 peptide and citrullinated peptide in positive patients. Results showed a statistical difference in RA patients (26% vs 43%, p=0.0170) supporting the hypothesis of the involvement of MAP in RA. These two virulence factors interfering with signal transduction in the host and are secreted proteins necessary for the survival of the pathogen in the harsh environment presented by the macrophage.33,36
The noteworthy result towards MAP citrullinate peptide suggests that it is likely that MAP infection may trigger a citrullination process in the attempt to eliminate the pathogen by self-feeding the autoimmune process. Significantly, by carrying out a bioinformatics analysis, PtpA and PknG proteins present in their primary sequence could be potentially citrullinated from the macrophage PAD after bacterial infection, triggering the inflammatory response ((PtpA (Protein tyrosine phosphatase A; UniProtKB Accession number: A0A200GPK8) and PknG (Protein kinase G; UniProtKB Accession number: A0A202FS53)). It has now been established that citrullination, together with carbamylation, is a process that, although physiologically present in nature, is more exacerbated in autoimmune diseases. Furthermore, they are a predictive marker of the onset of the disease. This is significant because of the silent stage of MAP when infecting cells in which the citrullination may occur, even the disease develops much later. Therefore, the next step in our research is to citrullinate these proteins and to evaluate the Ab response in subjects with active arthritis and those at onset in order to understand if citrullination is part of a process generated by the host to eliminate the pathogen. In future work, the reactivity towards PtpA and PknG in the different rheumatic diseases will be tested in order to analyze similarities and/or differences.
Moreover, to understand the binding of MAP infection with RA in detail, we plan to analyze the molecular pathways of macrophages in vitro and in mouse models of arthritis following MAP infection. This helps to understand what kind of molecules (cytokines and chemokines)42 are produced and how these can influence the activity of the other immune cells as the same macrophages and neutrophils.43–45 Furthermore, the analysis of expression of some important transcription factors in the regulation of the immune response, such as IRF5,46,47 JUNB,48 with the RNA sequencing (RNA-seq) technique and qPCR will allow for a better understanding for how MAP is involved in the etiopathogenesis of RA.
Conclusion
Our findings in this paper might stimulate further studies to verify our observations and open new frontiers for the investigation of pathogenesis of RA, including the possibility to develop new intervention strategies and the development of new drugs.
Acknowledgments
PORFSE Sardinia. “Finanziamento straordinario una tantum per la ricerca” 2019, Università di Sassari to Prof Sechi and Prof Passiu. Prof Bach contributed for the publishing fee with personal grant.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.McNees AL, Markesich D, Zayyani NR, Graham DY. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2015;9(12):1523–1534. doi: 10.1586/17474124.2015.1093931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuenstner JT, Naser S, Chamberlin W, et al. The consensus from the Mycobacterium avium ssp. paratuberculosis (MAP) Conference 2017. Front Public Health. 2017;5:208. doi: 10.3389/fpubh.2017.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feller M, Huwiler K, Stephan R, et al. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7(9):607–613. doi: 10.1016/S1473-3099(07)70211-6 [DOI] [PubMed] [Google Scholar]
- 4.Sechi LA, Dow CT. Mycobacterium avium ss. paratuberculosis Zoonosis – the hundred year war – beyond Crohn’s disease. Front Immunol. 2015;6:96. doi: 10.3389/fimmu.2015.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eslami M, Shafiei M, Ghasemian A, et al. Mycobacterium avium paratuberculosis and Mycobacterium avium complex and related subspecies as causative agents of zoonotic and occupational diseases. J Cell Physiol. 2019;234(8):12415–12421. doi: 10.1002/jcp.28076 [DOI] [PubMed] [Google Scholar]
- 6.Waddell LA, Rajić A, Stärk KD, McEWEN SA. The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: a systematic review and meta-analyses of the evidence. Epidemiol Infect. 2015;143(15):3135–3157. doi: 10.1017/S095026881500076X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawzy A, Zschöck M, Ewers C, Eisenberg T. Genotyping methods and molecular epidemiology of Mycobacterium avium subsp. paratuberculosis (MAP). Int J Vet Sci Med. 2018;6(2):258–264. doi: 10.1016/j.ijvsm.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sergeant ESG, McAloon CG, Tratalos JA, Citer LR, Graham DA, More SJ. Evaluation of national surveillance methods for detection of Irish dairy herds infected with Mycobacterium avium subspecies paratuberculosis. J Dairy Sci. 2019;102(3):2525–2538. doi: 10.3168/jds.2018-15696 [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama K, Cossu D, Hoshino Y, Tomizawa Y, Momotani E, Hattori N. Anti-mycobacterial antibodies in paired cerebrospinal fluid and serum samples from Japanese patients with multiple sclerosis or neuromyelitis optica spectrum disorder. J Clin Med. 2018;7:12. doi: 10.3390/jcm7120522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossu D, Yokoyama K, Tomizawa Y, Momotani E, Hattori N. Altered humoral immunity to mycobacterial antigens in Japanese patients affected by inflammatory demyelinating diseases of the central nervous system. Sci Rep. 2017;7(1):3179. doi: 10.1038/s41598-017-03370-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mameli G, Cocco E, Frau J, Marrosu MG, Sechi LA. Epstein-Barr virus and Mycobacterium avium subsp. paratuberculosis peptides are recognized in sera and cerebrospinal fluid of MS patients. Sci Rep. 2016;6:22401. doi: 10.1038/srep22401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slavin YN, Bo M, Caggiu E, et al. High levels of antibodies against PtpA and PknG secreted by Mycobacterium avium ssp. paratuberculosis are present in neuromyelitis optica spectrum disorder and multiple sclerosis patients. J Neuroimmunol. 2018;323:49–52. doi: 10.1016/j.jneuroim.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 13.Bo M, Niegowska M, Arru G, et al. Mycobacterium avium subspecies paratuberculosis and myelin basic protein specific epitopes are highly recognized by sera from patients with Neuromyelitis optica spectrum disorder. J Neuroimmunol. 2018;318:97–102. doi: 10.1016/j.jneuroim.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 14.Dow CT. M. paratuberculosis and Parkinson’s disease – is this a trigger. Med Hypotheses. 2014;83(6):709–712. doi: 10.1016/j.mehy.2014.09.025 [DOI] [PubMed] [Google Scholar]
- 15.Arru G, Caggiu E, Paulus K, Sechi GP, Mameli G, Sechi LA. Is there a role for Mycobacterium avium subspecies paratuberculosis in Parkinson’s disease? J Neuroimmunol. 2016;293:86–90. doi: 10.1016/j.jneuroim.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 16.Niegowska M, Rapini N, Piccinini S, et al. Type 1 diabetes at-risk children highly recognize Mycobacterium avium subspecies paratuberculosis epitopes homologous to human Znt8 and proinsulin. Sci Rep. 2016;6:22266. doi: 10.1038/srep22266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niegowska M, Wajda-Cuszlag M, Stępień-Ptak G, et al. Anti-HERV-WEnv antibodies are correlated with seroreactivity against Mycobacterium avium subsp. paratuberculosis in children and youths at T1D risk. Sci Rep. 2019;9(1):6282. doi: 10.1038/s41598-019-42788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cossu D, Yokoyama K, Sakanishi T, Momotani E, Hattori N. Adjuvant and antigenic properties of Mycobacterium avium subsp. paratuberculosis on experimental autoimmune encephalomyelitis. J Neuroimmunol. 2019;330:174–177. doi: 10.1016/j.jneuroim.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 19.Bo M, Erre GL, Niegowska M, et al. Interferon regulatory factor 5 is a potential target of autoimmune response triggered by Epstein-Barr virus and Mycobacterium avium subsp. paratuberculosis in rheumatoid arthritis: investigating a mechanism of molecular mimicry. Clin Exp Rheumatol. 2018;36(3):376–381. [PubMed] [Google Scholar]
- 20.Bo M, Niegowska M, Erre GL, et al. Rheumatoid arthritis patient antibodies highly recognize IL-2 in the immune response pathway involving IRF5 and EBV antigens. Sci Rep. 2018;8(1):1789. doi: 10.1038/s41598-018-19957-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 22.Isaacs JD. The changing face of rheumatoid arthritis: sustained remission for all? Nat Rev Immunol. 2010;10(8):605–611. doi: 10.1038/nri2804 [DOI] [PubMed] [Google Scholar]
- 23.Stahl EA, Raychaudhuri S, Remmers EF, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42(6):508–514. doi: 10.1038/ng.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki A, Yamada R, Chang X, et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat Genet. 2003;34(4):395–402. doi: 10.1038/ng1206 [DOI] [PubMed] [Google Scholar]
- 25.Olsen I, Singhrao SK, Potempa J. Citrullination as a plausible link to periodontitis, rheumatoid arthritis, atherosclerosis and Alzheimer’s disease. J Oral Microbiol. 2018;10(1):1487742. doi: 10.1080/20002297.2018.1487742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engström M, Eriksson K, Lee L, et al. Increased citrullination and expression of peptidylarginine deiminases independently of P. gingivalis and A. actinomycetemcomitans in gingival tissue of patients with periodontitis. J Transl Med. 2018;16(1):214. doi: 10.1186/s12967-018-1588-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd KA, Wigerblad G, Sahlström P, et al. Differential ACPA binding to nuclear antigens reveals a PAD-Independent pathway and a distinct subset of acetylation cross-reactive autoantibodies in rheumatoid arthritis. Front Immunol. 2019;9:3033. doi: 10.3389/fimmu.2018.03033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(8):472–485. doi: 10.1038/nrrheum.2016.91 [DOI] [PubMed] [Google Scholar]
- 29.Weiss M, Blazek K, Byrne AJ, Perocheau DP, Udalova IA. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators Inflamm. 2013;2013:245804. doi: 10.1155/2013/245804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saliba DG, Heger A, Eames HL, et al. IRF5: relA interaction targets inflammatory genes in macrophages. Cell Rep. 2014;8(5):1308–1317. doi: 10.1016/j.celrep.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaubey KK, Singh SV, Gupta S, et al. Mycobacterium avium subspecies paratuberculosis – an important food borne pathogen of high public health significance with special reference to India: an update. Vet Q. 2017;37(1):282–399. doi: 10.1080/01652176.2017.1397301 [DOI] [PubMed] [Google Scholar]
- 32.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 33.Bach H, Sun J, Hmama Z, Av-Gay Y. Mycobacterium avium subsp. paratuberculosis PtpA is an endogenous tyrosine phosphatase secreted during infection. Infect Immun. 2006;74(12):6540–6546. doi: 10.1128/IAI.01106-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia A, Stempak JM, Grist J, Bressler B, Silverberg MS, Bach H. Effect of inflammatory bowel disease therapies on immunogenicity of Mycobacterium paratuberculosis proteins. Scand J Gastroenterol. 2104;49(2):157–163. doi: 10.3109/00365521.2013.857713 [DOI] [PubMed] [Google Scholar]
- 35.Gurung RB, Begg DJ, Purdie AC, Bach H, Whittington RJ. Immunoreactivity of protein tyrosine phosphatase A (PtpA) in sera from sheep infected with Mycobacterium avium subspecies paratuberculosis. Vet Immunol Immunopathol. 2014;160(1–2):129–132. doi: 10.1016/j.vetimm.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 36.Bach E, Raizman EA, Vanderwal R, et al. Immunogenicity of PtpA secreted during Mycobacterium avium ssp. paratuberculosis infection in cattle. Vet Immunol Immunopathol. 2018;198:1–5. doi: 10.1016/j.vetimm.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 37.Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. [DOI] [PubMed] [Google Scholar]
- 38.Trouw LA, Rispens T, Toes REM. Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(6):331–339. doi: 10.1038/nrrheum.2017.15 [DOI] [PubMed] [Google Scholar]
- 39.Jung H, Jung SM, Rim YA, et al. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PLoS One. 2017;12(11):e0188698. doi: 10.1371/journal.pone.0188698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee A, Jantsch V, Khan R, et al. Rheumatoid arthritis-associated autoimmunity due to aggregatibacter actinomycetemcomitans and its resolution with antibiotic therapy. Front Immunol. 2018;9:2352. doi: 10.3389/fimmu.2018.02352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mameli G, Erre GL, Caggiu E, et al. Identification of a HERV-K env surface peptide highly recognized in Rheumatoid Arthritis (RA) patients: a cross-sectional case-control study. Clin Exp Immunol. 2017;189(1):127–131. doi: 10.1111/cei.2017.189.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–442. doi: 10.1038/nri2094 [DOI] [PubMed] [Google Scholar]
- 43.O’Neil LJ, Kaplan MJ. Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease. Trends Mol Med. 2019;25(3):215–227. doi: 10.1016/j.molmed.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 44.Weiss M, Byrne AJ, Blazek K, et al. IRF5 controls both acute and chronic inflammation. Proc Natl Acad Sci U S A. 2015;112(35):11001–11006. doi: 10.1073/pnas.1506254112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(10):593–601. doi: 10.1038/nrrheum.2014.80 [DOI] [PubMed] [Google Scholar]
- 46.Almuttaqi H, Udalova IA. Advances and challenges in targeting IRF5, a key regulator of inflammation. Febs J. 2019;286(9):1624–1637. doi: 10.1111/febs.2019.286.issue-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoyratty TE, Udalova IA. Diverse mechanisms of IRF5 action in inflammatory responses. Int J Biochem Cell Biol. 2018;99:38–42. doi: 10.1016/j.biocel.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 48.Moon YM, Lee SY, Kwok SK, et al. The Fos-related antigen 1-JUNB/activator protein 1 transcription complex, a downstream target of signal transducer and activator of transcription 3, induces T helper 17 differentiation and promotes experimental autoimmune arthritis. Front Immunol. 2017;8:1793. doi: 10.3389/fimmu.2017.01793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arsenault RJ, Maattanen P, Daigle J, Potter A, Griebel P, Napper S. From mouth to macrophage: mechanisms of innate immune subversion by Mycobacterium avium subsp. paratuberculosis. Vet Res. 2014;45(1):54. doi: 10.1186/1297-9716-45-54 [DOI] [PMC free article] [PubMed] [Google Scholar]