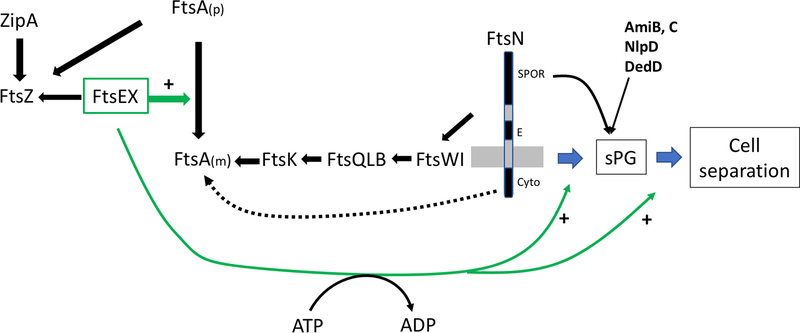
Fig. 1.
Roles of FtsEX in cell division. The Z ring consists of dynamic polymers of FtsZ attached to the membrane by ZipA and FtsA. Both proteins bind to the conserved C-terminal tail (CCTP) of FtsZ. FtsEX also interacts with the conserved CCTP of FtsZ and once at the Z ring, FtsEX, in a step that does not require ATP hydrolysis, acts on FtsA to promote formation of monomers which are active in recruiting the remaining divisome proteins that occurs in a sequential manner. The recruitment of FtsN, which triggers the initiation of sPG synthesis, involves interaction of the cyto domain of FtsN with FtsA and the interaction of the E domain of FtsN with the divisome in the periplasm. FtsN action leads to ATP hydrolysis by FtsEX and activation of FtsWI to synthesize sPG. It also leads to the recruitment of many other proteins including amidases (AmiB&C), an activator (NlpD of AmiC) and another stimulator of sPG (DedD). ATP hydrolysis by FtsEX also regulates EnvC, which is bound to the large periplasmic loop of FtsX, causing it to activate amidases in the periplasm leading to cell separation. Since FtsEX is required for activation of sPG synthesis and PG hydrolysis it couples these processes at the septum.