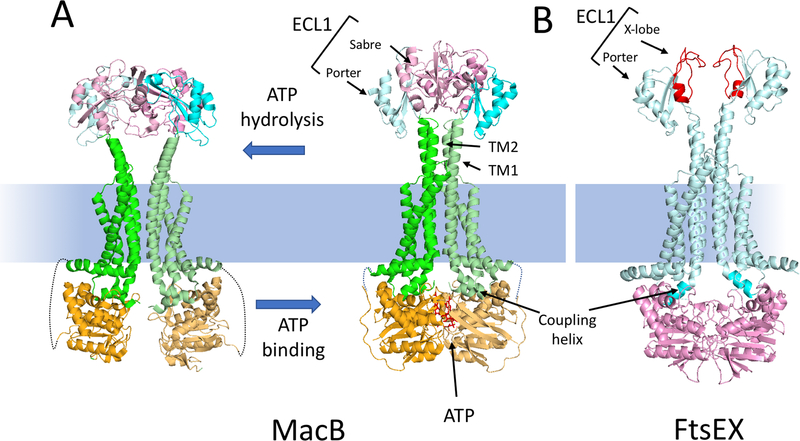
Fig. 2.
MacB as a model for the Type VII subfamily of ABC transporters and comparison to a model of FtsEX. (A) The structures of a MacB dimer in the nucleotide free form (5NIL) and the ATP bound form (5LJ7) are depicted. The NBD (light orange), the membrane component (green) and the large extracellular domain (ECL1) are depicted. ECL1 is split into two domains: porter (cyan) and sabre (pink). ATP binding brings the NBDs together causing the long TM1 and TM2 helices to form a tight helical bundle distal to the membrane causing conformational changes in the ECL1. The linkage between the NBD and the membrane component is not resolved in the structure and is indicated by a dotted line. (B) A model of the ATP form of FtsEX [59]; FtsE (pink) FtsX (cyan). The ECL1 of FtsX has a porter domain and a unique FtsX domain (called X-lobe [red]) instead of a sabre domain. This domain is responsible for interaction with the coiled coil region of its effector. The coupling helix of the TMD (FtsX) is inserted into a cleft of the NBD (FtsE).