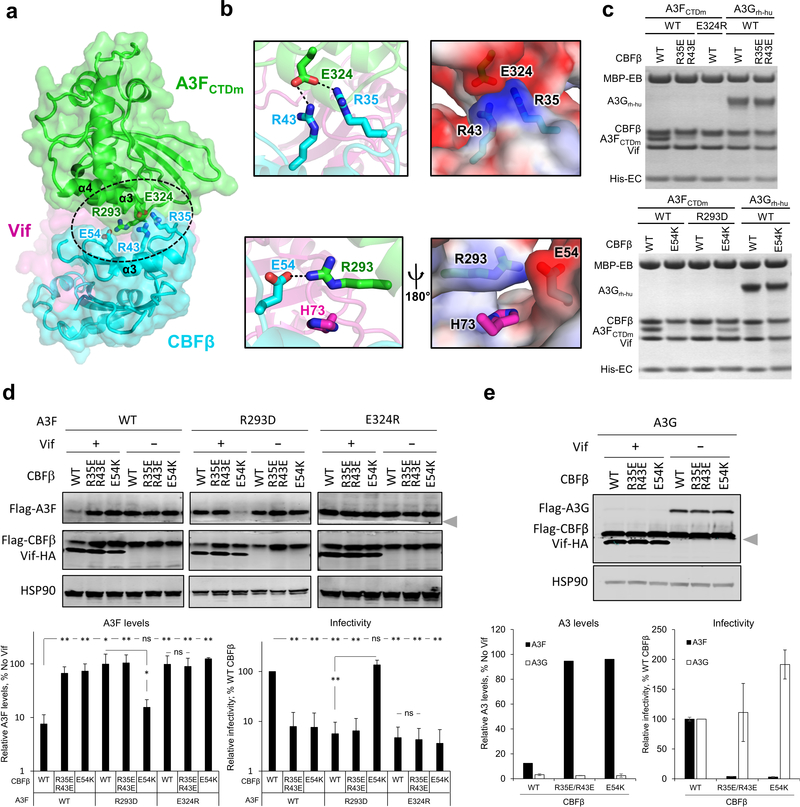
Fig. 2: The CBFβ-A3F interface is important for Vif–CBFβ–A3F complex formation, Vif-mediated A3F degradation, and viral infectivity.
a, Overview of the CBFβ-A3FCTDm interface (oval) with the critical interacting residues highlighted as sticks. The zinc atom is shown as red sphere. b, Detailed illustrations of the interacting residues and the electrostatic complementation at the interface. Blue, positively charged; red, negatively charged. c, Mutational analysis of the interactions by an in vitro binding assay using MBP-tagged Vif–CBFβ–EloB–EloC variants to pulldown A3FCTDm variants or A3Grh-hu. WT, wild type. The loading controls are shown in Supplementary Fig. 1a & c. d, Analysis of the critical interacting residues at the CBFβ-A3F interface in cell-based Vif-mediated A3F degradation and infectivity assays (mean ± s.d.; n=4 biologically independent experiments). The blot was cut as indicated (gray arrow), with one part probed for Flag-A3F and HSP90, and the other for Flag-CBFβ and Vif-HA. e, The A3F-interacting CBFβ residues are not critical for Vif-mediated A3G degradation (mean ± s.d.; n=3 biologically independent experiments for A3G). The blot was analyzed as in d; the Flag-CBFβ band was partially cut and could be detected in both parts by the same anti-Flag antibody. Statistical significance of A3 degradation and infectivity was assessed by two-tailed t-test assuming equal variance; *, P < 0.05; **, P < 0.005; ns, p-value not significant. Uncropped images and data behind graphs are available as Source Data.