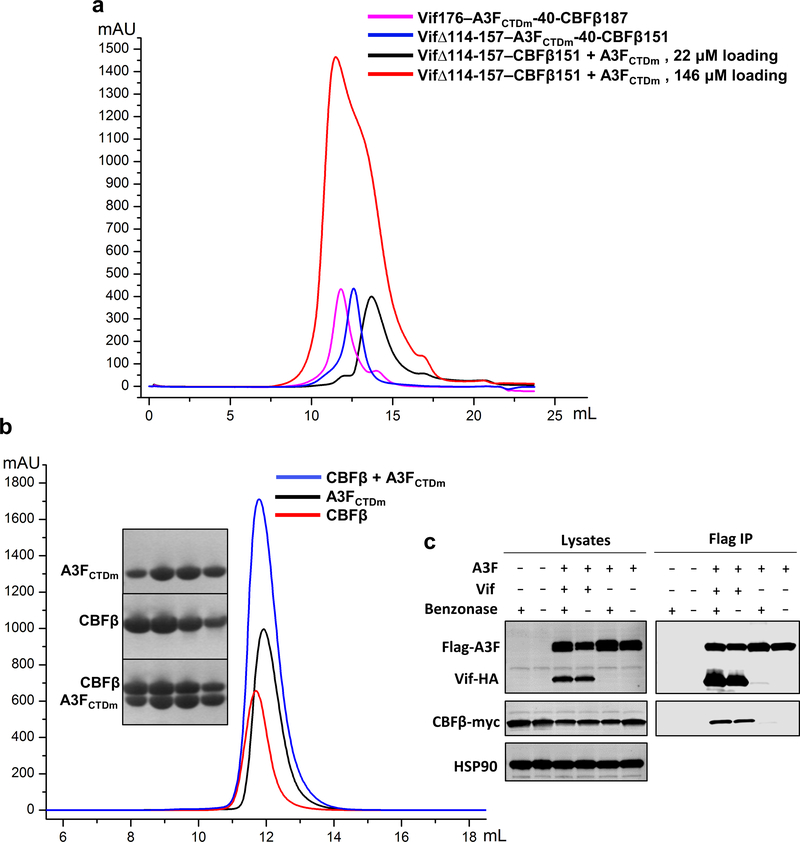
Extended Data Fig. 1. Biochemical and cellular characterizations of various Vif–CBFβ–A3FCTDm assemblies and the A3FCTDm-CBFβ interaction.
a, The Vif–CBFβ–A3FCTDm fusion complex with or without the Vif α-domain and corresponding interacting CBFβ C-terminus stays as tetramer in low salt solution. The unfused Vif–CBFβ–A3FCTDm without these regions switches from monomer to tetramer at high protein concentration (146 μΜ loading concentration). b, No obvious shift for the elution peak was observed upon incubation of CBFβ and A3FCTDm compared to the CBFβ alone or A3FCTDm alone. The SDS-PAGE analysis of the peak fractions of CBFβ alone, A3FCTDm alone and CBFβ/A3FCTDm mixture is indicated. c, Co-immunoprecipitation (Co-IP) analysis of the interaction between A3F and CBFβ in the presence or absence of Vif in cells. Flag-A3F and CBFβ-myc were co-transfected with or without Vif-HA and co-immunoprecipitated using anti-Flag antibody. In the absence of Vif, no binary A3F and CBFβ binding was observed. A representative blot from two independent experiments was shown.