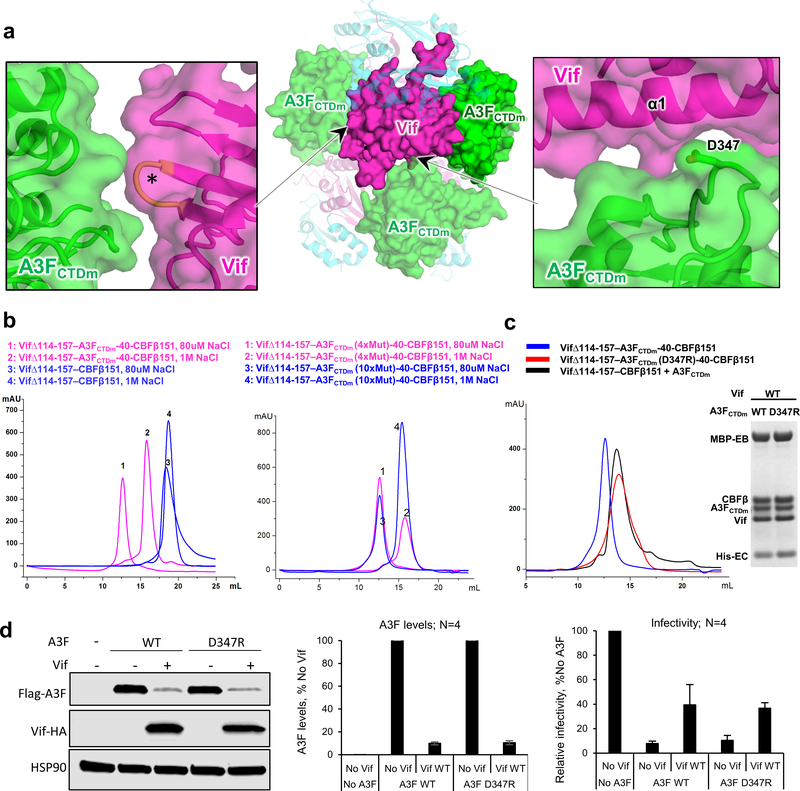
Extended Data Fig. 4. Effects of Vif–CBFβ–A3FCTDm tetramer on A3F-Vif interaction, Vif-mediated A3F degradation, and viral infectivity.
a, Inter-ternary complex interfaces between Vif and A3FCTDm detected in the tetrameric complex. Center, Overview of the two inter-ternary complex interfaces (pointed to by the arrows) involving one Vif molecule. Vif is shown in magenta, CBFβ in cyan, and A3FCTDm in green. One ternary complex containing the major Vif-A3F interface is marked by an oval. The detailed illustrations of the two interfaces are shown on the sides, with one involving the Vif α1 helix (right), and the other involves Vif residues (orange, marked by *) in the 55VxIPLx4–5L64 motif (left). b, SEC binding assays show that the tetramer formation depends on the presence of A3FCTDm (left) but not on the solubility-enhancing mutations of A3FCTDm (right). In contrast to the Vif–CBFβ–A3FCTDm complex which switched from monomer to tetramer when reducing salt concentration, Vif-CBFβ alone stayed as monomer. Six A3FCTDm residues (196, 247, 248, 310, 314, 315) located near the observed tetramer interfaces were reverted back to WT amino acids to verify that this A3FCTDm variant (with 4 remaining point mutations away from the interface and without any disturbance to A3F structure) retained the ability to form tetramers. c, SEC binding (left) and MBP pull-down (right) assays show that the A3FCTDm D347R mutation disrupts the tetramer formation but not the individual Vif–CBFβ–A3FCTDm ternary complex. The loading controls are shown in Supplementary Fig. 1b & c. d, The effect of D347R mutation on A3F sensitivity to Vif-mediated degradation indicated by western blot (left), quantified A3F levels relative to No Vif (mean ± s.d.; n=4 biologically independent experiments; middle), and relative infectivity (mean ± s.d.; n=4 biologically independent experiments; right). A3FD347R retained WT A3F-like sensitivity to Vif-mediated degradation, which resulted in rescue of viral infectivity.