Figure 1.
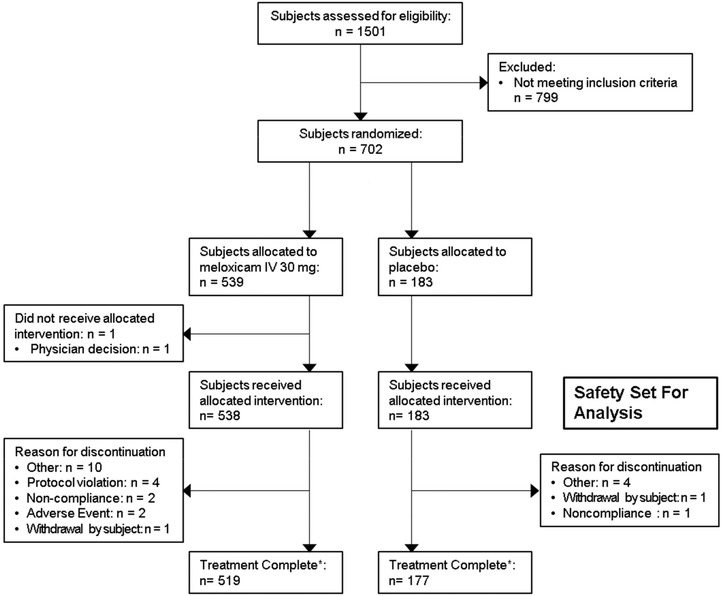
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patient disposition in study NCT02720692. IV indicates intravenous. *All treated subjects were to complete a final safety assessment by telephone 28 days following their last study dose; 8 subjects allocated to meloxicam IV 30 mg, and 3 subjects allocated to placebo were lost to follow‐up.
