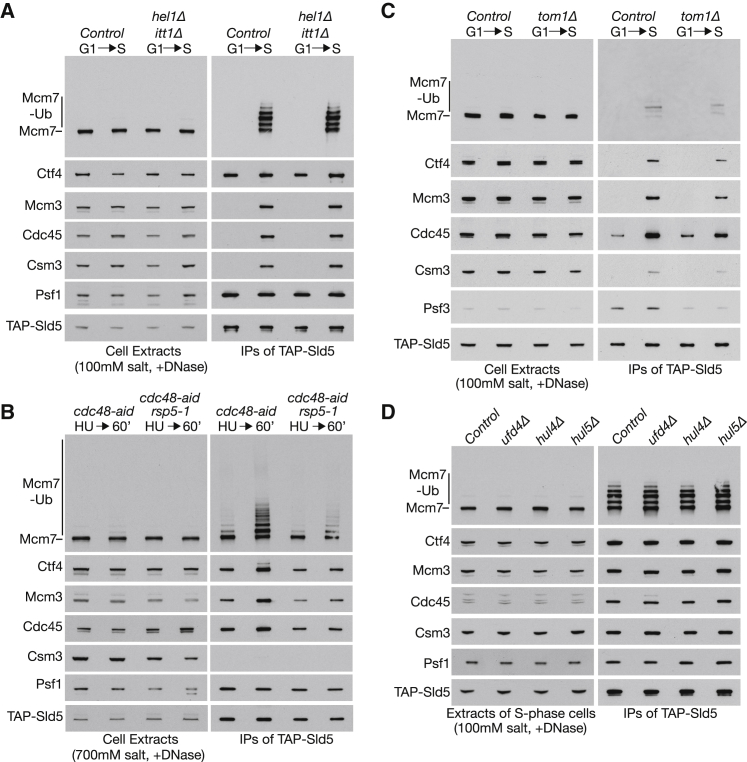
Figure 1.
RBR and HECT E3 Ligases Are Dispensable for CMG Ubiquitylation in Budding Yeast
(A) TAP-SLD5 control cells (YSS47) and TAP-SLD5 hel1Δ itt1Δ cells (YPM141) were grown at 24°C and synchronized in G1 phase by addition of mating pheromone before release into S phase for 30 min. Samples were used to make “low-salt” cell extracts (100 mM KOAc), in which in vitro CMG ubiquitylation was monitored by immunoprecipitation of the TAP-tagged Sld5 subunit of GINS. The indicated proteins were monitored by immunoblotting. DNA content was also monitored by flow cytometry (see Figure S1A).
(B) TAP-SLD5 (YMM228) and TAP-SLD5 rsp5-1 (YPM174) cells were synchronized in G1 phase at 24°C and then released into fresh medium containing 0.2M hydroxyurea (HU) to arrest cells in early S phase. The cultures were then shifted to 37°C for 60 min in the presence of 500 μM indoleacetic acid (auxin) in order to inactivate Rsp5-1 and deplete Cdc48-aid. The cells were subsequently released for 60 min at 37°C into fresh medium lacking HU but containing auxin. “High-salt” extracts were made in the presence of 700 mM KOAc in order to inhibit in vitro CMG ubiquitylation (Maric et al., 2014) and thus reveal in vivo ubiquitylation. After digestion of chromosomal DNA, TAP-Sld5 was isolated by immunoprecipitation and the associated factors monitored as above.
(C) TAP-SLD5 (YSS47) and TAP-SLD5 tom1Δ (YPM44) cells were grown and processed as in (A).
(D) TAP-SLD5 (YSS47), TAP-SLD5 ufd4Δ (YPM150), TAP-SLD5 hul4Δ (YPM192), and TAP-SLD5 hul5Δ (YPM195) cells were treated as above (in this case only the S phase samples are shown).
See also Figure S1.