Figure 3.
A Complex of SCFDia2 with the Replisome Supports In Vitro Ubiquitylation of the CMG Helicase by Cdc34
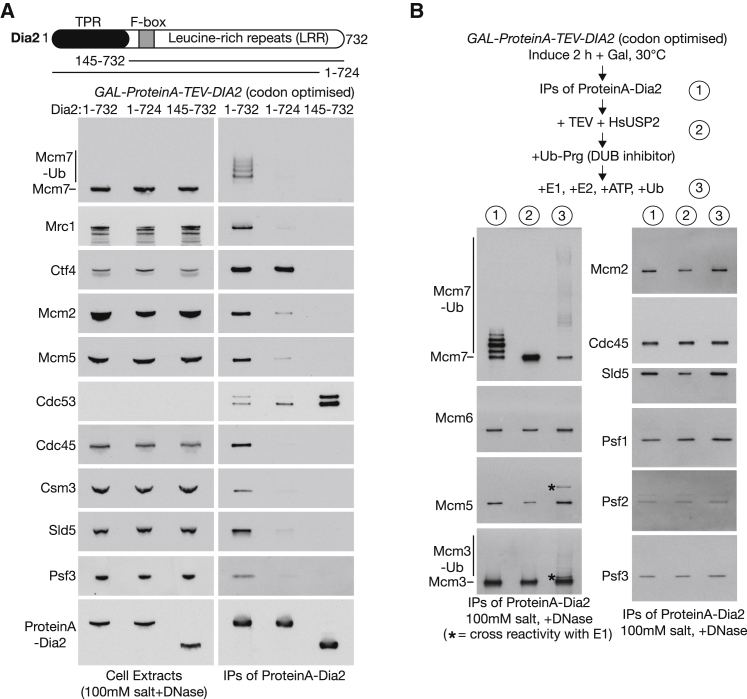
(A) Asynchronous cultures of cells with ProteinA-tagged versions of Dia2 1–732 (full-length Dia2; YTM418), Dia2 1–724 (deletion of Dia2 TPR domain; YPM220), or Dia2 145–732 (small truncation in Dia2 LRR domain; YTM495) under the control of the GAL1,10 promoter were grown at 30°C in medium containing raffinose before induction for 2 h in medium containing galactose. The tagged proteins were then isolated from cell extracts by immunoprecipitation on IgG-coupled beads. Half of each sample was treated with TEV protease to release bound proteins from the beads (to avoid interference of TAP-tagged protein in immunoblots of similarly sized proteins), whereas the other half was analyzed by boiling directly in Laemmli buffer before SDS-PAGE.
(B) ProteinA-tagged full-length Dia2 (from YTM418) was isolated as above (sample 1) before release from beads with TEV protease and deubiquitylation with HsUSP2 for 1 h at 24°C (sample 2). After treatment for 15 min with the DUB inhibitor Ubi-Prg, samples were treated for a further 30 min at 24°C with 30 nM Uba1 E1 enzymes, 150 nM Cdc34 E2 enzyme, 50 μM ubiquitin, and 2.5 mM ATP. The indicated proteins were then monitored by immunoblotting.
See also Figures S1, S3, and S4.