Figure 3.
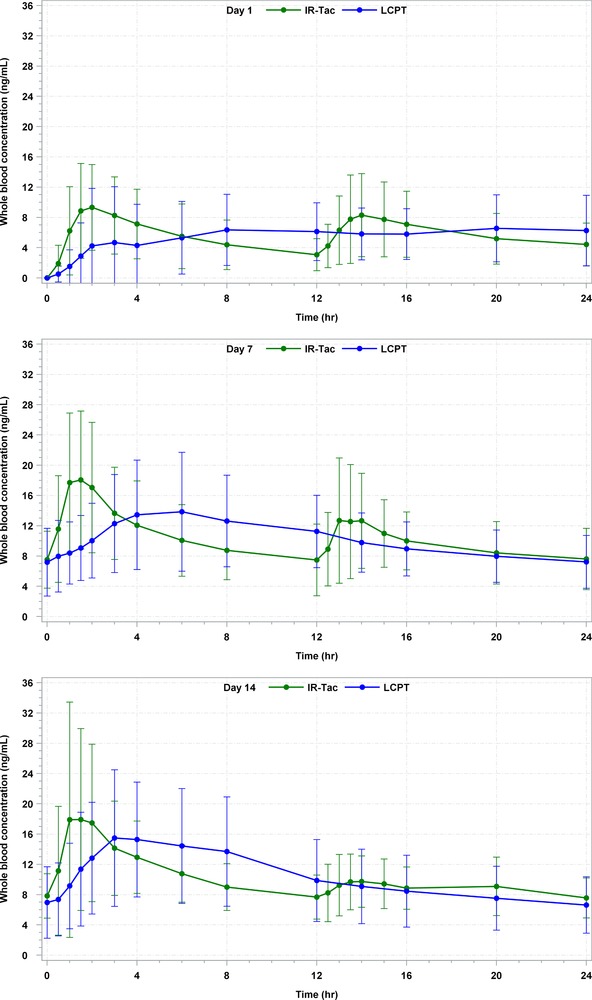
24‐hour arithmetic mean whole blood tacrolimus concentrations (ng/mL) and SD on days 1, 7, and 14 for LCPT and IR‐Tac, exposure normalized.* All BLQ values entered as zero and included as such in the calculation of means. BLQ, below limit of quantification; IR‐Tac, twice‐daily immediate‐release tacrolimus capsules; LCPT, once‐daily extended‐release tablet formulation of tacrolimus; SD, standard deviation.
*Exposure normalization is an adjustment by which the AUC of LCPT and IR‐Tac are made equal, and all other pharmacokinetic parameters mathematically adjusted, to allow direct comparisons of pharmacokinetic parameters under assumption of equivalent exposure.
