Abstract
Background
Few epidemiological data on autism spectrum disorders (ASD) exist for Arabic countries. We conducted the first survey of ASD in Qatar, a population with high consanguinity level.
Methods
This cross‐sectional survey was conducted from 2015 to 2018 in Qatar school‐age children (N = 176,960) from national and immigrant families. Children diagnosed with ASD were identified through medical centers and special needs schools. Records were abstracted and supplemented by parental interviews. Additionally, children attending 93 schools were screened; ASD case status was confirmed in random samples of screen‐positive and screen‐negative children. Prevalence was estimated after taking into account different sampling fractions and participation rates at each survey phase.
Results
One thousand three hundred and ninety‐three children already diagnosed with ASD were identified. Among 9,074 school survey participants, 760 screen‐negative children and 163 screen‐positive children were evaluated; 17 were confirmed to have ASD including five children newly diagnosed. Prevalence was 1.14% (95% CI: 0.89–1.46) among 6‐ to 11‐year‐olds. ASD was reported in full siblings/extended relatives in 5.9% (95% CI: 0.042–0.080)/11.8% (95% CI: 0.095–0.146) families. First‐degree consanguinity in Qatari cases (45%) was comparable to known population levels. Among 844 ASD cases (mean age: 7.2 years; 81% male), most children experienced language delay (words: 75.1%; phrase speech: 91.4%), and 19.4% reported developmental regression. At the time of the survey, persisting deficits in expressive language (19.4%) and peer interactions (14.0%) were reported in conjunction with behavioral problems (ADHD: 30.2%; anxiety: 11.0%). In multivariate logistic regression, ASD severity was associated with parental consanguinity, gestational diabetes, delay in walking, and developmental regression.
Conclusions
ASD prevalence in Qatar is consistent with recent international studies. The methods employed in this study should help designing comparable surveys in the region. We estimated that 187,000 youths under age 20 have ASD in Gulf countries. This figure should assist in planning health and educational services for a young, fast‐growing population.
Keywords: Arabic, autism spectrum disorders, epidemiology, screening, prevalence, child, school age, consanguinity, regression
Introduction
Autism spectrum disorder (ASD) is characterized by pervasive impairments in social reciprocity, communication, stereotyped behaviors, and restricted interests (APA, 2013). Reviews concluded that best estimates for ASD prevalence are around 1% (Elsabbagh et al., 2012; Meyers, Presmanes Hill, Zuckerman, & Fombonne, 2018), with some studies yielding estimates over 2% (Kim et al., 2011; Pelly, Vardy, Fernandez, Newhook, & Chafe, 2015; Randall et al., 2016). Four surveys in Arabic countries relied on unreliable methods (see Table S1). There is no standardized methodology to conduct ASD surveys, and variation in reported prevalence reflects, at least in part, this lack of standardization. ASD prevalence has increased steadily, but the interpretation of this trend remains uncertain (Fombonne, 2009). Survey methods variability, changes in diagnostic concepts and criteria, diagnostic substitution, improved identification, and awareness contributed to it (Hill, Zuckerman, & Fombonne, 2014). Yet, an increase in ASD incidence could also account for a portion of the upward prevalence trend; if so, it could point to environmental risk factors in ASD etiology operating with or in addition to well‐established genetic factors (Willsey & State, 2015; Woodbury‐Smith & Scherer, 2018). Consequently, monitoring ASD prevalence has become a public health priority in many countries.
Case ascertainment in earlier studies relied on identifying children already diagnosed with ASD. Improvement on this methodology was brought about in the US Centers for Disease Control and Prevention (CDC) surveillance program (Yeargin‐Allsopp et al., 2003). The CDC methodology screens the child population age 8; medical or educational records containing evidence of a social ‘trigger’ are abstracted whether or not an ASD diagnosis is already reported. An expert panel subsequently derives a best estimate diagnosis (Van Naarden Braun et al., 2007). Limitations to this methodology include an absence of surveys of children without services contacts and of direct diagnostic evaluation (Fombonne, 2018). The addition of a school survey in recent studies (Fombonne et al., 2016; Kim et al., 2011) has addressed one of these limitations. However, variability in methods and participation resulted in new challenges; despite applying appropriate weights to account for different sampling and participation rates at each survey phase, unchecked assumptions may have biased upwards prevalence estimates (Fombonne, 2018; Pantelis & Kennedy, 2015).
Most surveys have been conducted thus far in Western countries (Elsabbagh et al., 2012). World populations differ for economic wealth, nutritional status, maternal and child health, and genetic background as well as rates of consanguinity. Arabic countries have among the highest rates of consanguineous marriages (20%–50%; Tadmouri et al., 2009) that increase rates of homozygotes for recessive disorders and that may modulate the population risk of autism. Risk of ASD has not been studied in relation to population level of consanguinity. We conducted the first prevalence study of ASD in Qatar, a country with frequent and increasing first‐cousin marriages. The main objectives were (a) to assess the prevalence of ASD among school‐age children; (b) to describe correlates and developmental characteristics of children with ASD.
Methods
Site
Qatar is one of the Gulf Cooperation Council countries (GCC, including Saudi Arabia, Bahrain, United Arab Emirates, Oman, and Kuwait), with a population of 2.7 million (26% under age 25) (Ministry of Development Planning & Statistics, 2018a,2018b,2018c). The literacy rate is high (>96%) (UNICEF, 2016). School attendance is mandatory at age 5 and free (Ministry of Education, 2016). Free healthcare is available for nationals and residents, mostly delivered by the Hamad Medical Corporation (HMC, [Link]). Consanguineous marriage is frequent (>50%) in Qatar and other GCC countries (Bener & Alali, 2006).
Special provisions exist for individuals with disabilities. Preschoolers are referred to Child Development Center at HMC (CDC‐HMC). ASD is a separate eligibility special education category, and school‐age children are evaluated by the Roaa’ Center for Evaluation and Consultation (RCEC) funded by the Ministry of Education. After a multidisciplinary evaluation, RCEC issues individualized education plans shared with the parents. Students suspected to have ASD are referred to the CDC‐HMC or the Shafallah Center for a comprehensive medical and diagnostic assessment. The ASD diagnostic teams in both HMC and Shafallah Center comprise developmental pediatricians, psychologists, general pediatricians, speech pathologists, behavioral analysts, and therapists. All diagnosticians are trained to the Autism Diagnostic Interview – Revised (ADI‐R) (Rutter, LeCouteur, & Lord, 2003) and the Autism Diagnostic Observational Schedule (ADOS) (Lord, Rutter, DiLavore, & Risi, 2002). Based on family preference, assessments are conducted in Arabic or English by bilingual staff, and when needed, in another language with a translator.
Every public school includes special education staff and a coordinator office (Alshaban, 2018). Educational support for students with ASD is free. Reflecting various degrees of impairment, education for students with special needs ranges from full to partial integration within mainstream classrooms to attendance of special education classes or centers.
Sample selection
The target population was 5‐ to 12‐year‐old children born between January 1, 2005, and December 31, 2012, residing in Qatar (Qatari or expatriates). The total population was divided into two separate strata. First, through systematic screening of records at each data source, we identified all children who had been in contact with health or educational services for developmental, medical, behavioral, or learning problems and diagnosed with ASD (Qatar Clinical Centers; QCC). Second, we undertook a survey of the general population of children meeting inclusion criteria (Qatar School Survey; QSS).
Case finding methods
Qatar School Survey
Each school has its own database which includes students’ names, sex, date of birth, and address.
Screening phase
The Ministry of Education issued a letter encouraging each school participation to the survey. The team contacted 216 primary schools, and 172 were visited. Of these, 39 schools did not participate, 40 schools expressed an initial interest but later opted out mostly due to shortage of supportive staff, and 93 schools (N = 62,011 students) were finally included. Meetings were organized with staff and sometimes families to explain the study protocol. A school‐based coordinator established the list of students between the ages of 5 and 12 years attending grades 1–6. Children aged 5 attending kindergarten were not surveyed. Envelopes containing the screening instrument, a letter, a brochure about ASD and the consent letter were sent to parents and one reminder sent 2 weeks later. Screening questionnaires were scored, and participants subsequently ascribed to the ‘screen‐negative group’ or ‘screen‐positive group’ (see Figure 1) depending on whether or not their total score was below or above the previously validated threshold (see below).
Figure 1.
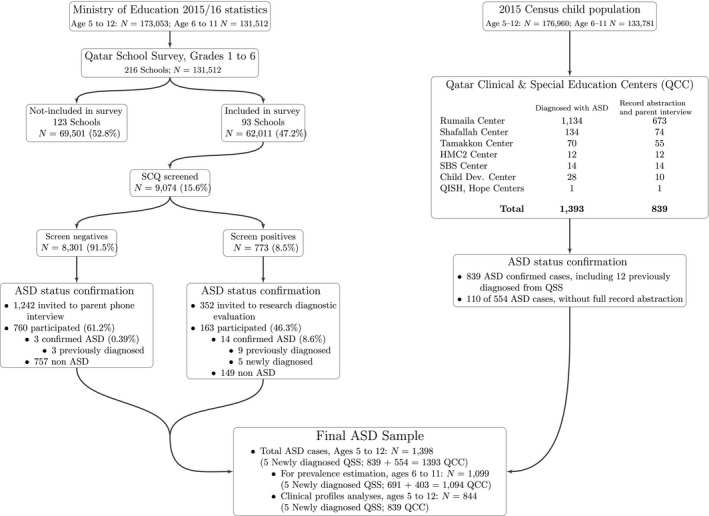
Qatar autism survey flowchart
Diagnostic confirmation phase
Parents from both screening groups were contacted by phone at least five times. In 182 cases, families could not be reached (incorrect/disconnected number). A family was considered to be a definite refusal when parents declined participation or could not be reached.
Screen‐negative (screen−) group
A brief semistructured telephone interview (QSS‐PTI; see below) was administered to rule out ASD in their child; when descriptions were ambiguous or suggestive of autism‐like symptoms, parents were invited to an in‐person evaluation. We randomly selected and contacted 1,242 participants (15% of the 8,301 screen). We kept inviting parents up to the point where about 10% of the screen− group could be evaluated. Overall, 760 (61.2%; 9.8% of all screen−) completed the QSS‐PTI.
Screen‐positive (screen+) group
Parents were invited to a direct assessment with their child; whenever this was not possible (distance, scheduling difficulty, parent preference), we used instead the QSS‐PTI. We kept inviting parents up to the point where at least 20% of participants could be evaluated. Of 352 families invited, 163 participated (46.3%; 21.1% of the 773 screen+). In 112 participants (68.7% of the 163 evaluated screen+), ASD could be confidently ruled out after QSS‐PTI completion. The remaining 51 families (31.3% of the 163 evaluated screen+) participated in in‐person evaluations; all evaluations but one were conducted in QBRI research offices (one at school).
Qatar Clinical Centers
QCC sites
Children already diagnosed with ASD were identified at eight sources (for site details please see Appendix S1 in Supporting Information).
Data collection
A total of 1,393 unique children were retained in the QCC sample. During the 4 years of the survey, we located and abstracted medical/educational records for 839 cases. Resource limitations did not allow for data abstraction on the remaining 554 children. However, on a subsample (N = 110; 19.9%), we confirmed the accuracy of the ASD diagnosis with the clinical team. Consistent with the 839 abstracted cases, mean age was 6.8 years (SD = 1.59) and there were 79.4% boys. Diagnosis had been established via multidisciplinary assessment using the ADOS (Lord et al., 2002) and a structured clinical evaluation in 51 cases (46.4%) and only structured clinical diagnosis in the remaining 59 (53.6%).
For the 839 cases, we supplemented and updated the record information with current parental reports. In 108 cases (12.9%), parents could not be contacted. For the remainder 731 cases, 5 (0.7%) refused to participate (but consented to record abstraction), and 235 direct interviews and 491 telephone interviews were performed.
Instruments
QSS screening phase: Social Communication Questionnaire (SCQ)
We used the Lifetime version of the SCQ (Rutter, Bailey, & Lord, 2003) that evaluates participants’ developmental history and current behaviors (either Arabic or English version). It comprises 40 questions with ‘yes’ or ‘no’ responses that can be answered in <10 min. Each item is scored as 0 or 1, and the sum of 39 item scores yields a total SCQ score ranging from 0 to 39. In the original validation, cutoffs of 15 and 22 had been proposed to identify children with a broad or narrow form of ASD (Berument, Rutter, Lord, Pickles, & Bailey, 1999). A cutoff of 12 was also proposed to optimize SCQ performance in population‐based samples (Eaves, Wingert, Ho, & Mickelson, 2006).
In our pilot study (Aldosari et al., 2019), we recruited 412 children [73.8% males; mean age: 8.5 years (SD = 2.6); 206 with ASD, 206 typically developing (TD)]. The mean SCQ total score was significantly higher in the ASD group (mean difference: 13.8 points; p < .0001) and showed excellent discrimination between ASD and TD children (Area Under the Curve = 0.95; 95% CI: 0.93–0.97). The cutoff of 15 achieved an optimal tradeoff between sensitivity and specificity and was subsequently employed to define screened positive or negative status in the QSS.
QSS diagnostic confirmation phase
To establish ASD case status, we relied either on a telephone interview or an in‐person diagnostic assessment.
Semistructured telephone interview
The QSS Parental Telephone Interview (QSS‐PTI) covered basic socio‐demographic data, and screening questions about current and/or past presence of communication, social relationships, atypical behaviors consistent with DSM 5 diagnostic criteria, disruptive or emotional problems, learning or social difficulties, and other developmental abnormalities. Additional questions about current peer relationships and teacher concerns about child's adjustment at school were asked. The interview lasted 20–30 min. All interviewers were well experienced in conducting clinical interviews with caregivers of children with ASD. Behavioral descriptions were written down for later coding. The presence/absence of each other problem area was rated as present (=1) or absent (=0). A rating of 1 meant that significant symptoms associated with impaired functioning were evidenced. Children with no teacher and parent concern, normal peer relationship and no evidence of autistic symptom were ruled out for ASD. Children with evidence of such symptoms in combination with impairment in at least one area of functioning were selected for the next stage.
In‐person diagnostic assessment
These assessments (duration: ≈2.5 hr) were conducted by at least two of four members of the research team, all with ASD clinical experience and trained to the ADI‐R (Rutter, LeCouteur et al., 2003; Rutter, Bailey et al., 2003) and ADOS‐2 (Lord et al., 2002). One staff member interviewed caregivers while another one evaluated the child through direct observation and the ADOS‐2 (Lord et al., 2002) when needed.
QCC – Abstraction Form (QCC‐AF)
The QCC‐AF was developed to abstract clinical information deriving from records and parental interviews. It contains sections on socio‐demographics, medical and developmental history, comorbid medical, behavioral and genetic conditions, past/current educational and service use. Country of origin was coded in four categories: Qatar, other Gulf countries, other Arabic countries, and other non‐Arabic countries. Parental ages were used as continuous variables and also grouped in 6‐year bands (collapsed in three for some analyses). Parental education was coded on four levels as follows: Primary school, Secondary school, College level, and Master degree or higher (collapsed in three for some analyses). Consanguinity was considered present for parents who were first‐degree or second‐degree cousins; only first‐degree consanguinity was retained for analyses on severity. Recurrence of ASD was separately coded for siblings (full and half) and for extended relatives. Low birthweight (BW) was calculated as BW < 2,500 g. Motor delay was defined as delay in Sitting (≥8 months) or in Walking (≥18 months). Language delay in early childhood was defined as Delay in first use of words (≥24 months) and Delay in phrase speech (≥33 months), similar to definitions used in the ADI‐R (Rutter, LeCouteur et al., 2003) that also relies on retrospective parental reporting. Current level of receptive language was coded in two categories: Good to Excellent, and Poor to Limited. All other variables were coded as Present (=1) or Absent (=0). For gestational maternal disease, obstetric complications, developmental regression involving loss of language or other skills, we created summary variables including any event in the underlying category. Data missing for over 25% of the sample (e.g. results of baseline medical workup, cognitive testing scores) are not reported.
We generated a composite severity score (CSS) by combining information on current functioning. Specifically, a score of 0 for no/lesser impairments and one for highest impairment was attributed to six items assessing the following: Receptive language, Expressive language, Reduced peer interactions, Academic performance difficulties, Requires behavioral support at school, Any emotional/behavioral problem. Item scores were summed into a composite ranging from 0 to 6 for each subject. The CSS sample mean was 1.0 (SD = 1.05; median = 1.0). The CSS distribution significantly departed from the normal distribution (Shapiro–Wilk test: p < .0001), and no statistical transformation could restore normality; thus, the CSS was dichotomized and analyzed categorically. Two fifths of the children (38.2%) had a CSS of 0, and 36.1% had a score of 1; these children were combined into the same low impairment category. The remaining children (25.7%) had a CSS score of 2 (17.1%), 3 (5.8%), or ≥4 (2.8%) and were combined into a high‐impairment category.
Final case determination
All available data were reviewed by the research team and a final diagnosis confirmed. Case confirmation was guided by DSM 5 (APA, 2013) diagnostic criteria for ASD although uneven levels of information did not allow for reliable scoring of individual diagnostic criteria. Diagnoses of Pervasive Developmental Disorder previously made in DSM‐IV‐TR (APA, 2000) were verified and converted to DSM 5 (APA, 2013) ASD diagnoses.
Ethical approval
Ethical approval was obtained from the QBRI and HMC Institutional Review Boards.
Statistical analyses
Qatar Clinical Centers‐AF data were analyzed in SPSS (IBM Corporation, Armonk, NY), and conventional statistical tests for categorical (Fisher's exact test; chi‐square) and continuous variables (t tests, Pearson or Spearman correlation coefficients) were used. Binary and multivariate logistic regression was used to evaluate predictors of binary variables. Variables with high (>25%) levels of missing data were excluded, and no imputation technique was used to adjust for variable‐ or item‐level missingness. In line with current recommendations from epidemiologists and biostatisticians (Perneger, 1998; Rothman, 2014), we did not use Bonferroni's adjustment for multiple tests. Throughout, a p‐value of .05 was therefore retained as level of statistical significance.
Prevalence calculations estimated two mutually exclusive prevalence proportions that were summed to generate a total population prevalence. The first prevalence figure was for already‐diagnosed cases, and the second prevalence estimate was for newly diagnosed cases. Because the primary schools survey focused on grades 1–6 and did not consistently sample children age 5 (still in Kindergarten) or 12 (already in middle school), we restricted the study population to 6‐ to 11‐year‐olds for the purpose of prevalence calculations. The prevalence for already‐diagnosed cases was arrived at by dividing the total number of cases ages 6–11 identified through the QCC component of the survey (see Figure 1) by the population size of residents aged 6–11 (N = 133,781) as per the national census from April 2015 (Ministry of Development Planning & Statistics, 2015).
The prevalence for newly diagnosed ASD cases reflected the results of the QSS component of the survey as a weighted average of the screen‐positive and screen‐negative groups. To execute the weighting and to estimate confidence intervals that reflected the level of uncertainty due to sampling variation, we simulated the sampling process from a hypothetical population based on the observed results in the QSS. The number of children with negative and positive screening results who participated in diagnostic status confirmation were simulated from binomial distributions with parameters n (number of trials) and p (probability of ‘success’) set to the observed sizes and participation rates of the screen+ and screen− groups. From the resulting numbers, we simulated newly diagnosed cases with probability corresponding to the observed results (number of newly diagnosed cases/number of screen+ or screen−, respectively). Where this number equaled zero, we added a small correction factor (n = 0.5) to the numerator so that the simulations would vary from zero. The prevalence of new diagnoses in each of the two groups was calculated. To translate these specific screening‐phase results prevalence estimates into a population estimate, we also simulated the first phase of the screening process, using the screen+ and screen− proportions as binomial probabilities and the number of children screened as the number of trials. We applied our estimated prevalence from the previous step to find the expected number of cases in each screening group. These served as the numerator for a final prevalence estimate, with the number screened as the denominator. We repeated this simulation 10,000 times. We used the mean prevalence from this simulation as the point estimate and the 2.5th and 97.5th percentiles as the bounds of the 95% confidence interval. Prevalence estimation was performed with Stata software, version 15 (StataCorp, 2017).
The total population prevalence was obtained by summing the prevalence proportions estimated for already‐diagnosed (QCC) and newly diagnosed (QSS) cases. To obtain estimates of precision, we simulated the expected number of cases from the QCC from a binomial distribution with the census population as the number of trials. We used our previously simulated prevalence estimates to obtain the expected number of newly diagnosed cases in the entire school‐age population (prevalence*population). We then took the 2.5th and 97.5th percentiles of the resulting prevalence estimates over 10,000 simulations as the 95% confidence interval. To obtain age‐ and gender‐specific estimates, we applied the age and gender distributions from the QCC to our simulated cases and used population estimates as denominators. Because there were too few cases newly identified through the school survey, age and gender prevalence calculations could not be reliably adjusted for the specific gender and age distribution observed among the few new diagnoses within the QSS; thus, we defaulted to the QCC age and sex distribution for the QSS prevalence components.
We expected to identify in the QSS children already diagnosed and ascertained in the QCC. This provided a unique opportunity to generate a second estimate for the prevalence of already‐diagnosed cases, this time derived from the school survey. We used the same statistical approach as that (described above) employed for the newly diagnosed cases in the QSS. We hypothesized that the two estimates generated with different methods should converge reasonably.
Other study features
Results
Qatar School Survey
Screening phase
A total number of 9,074 SCQ were returned by parents. The mean total SCQ score was 6.8 (SD = 5.3) (Table 1); 771 children (8.5%) scored at or above the cutoff. Gender was associated with screening status (p < .003) with 9.6% of boys and 7.8% of girls scoring above the SCQ cutoff. Mean SCQ total scores increased significantly with age in the entire sample (F = 15.2; p < .001) as well as in boys and girls separately (p < .001). The proportion of screen‐positive children increased from 6.4% among 5‐ to 6‐year‐olds to 10.8% among 11‐ to 12‐year‐olds (p < .001). The age trend was the same in girls and boys (Table 1), making it unlikely that the higher proportion of screen‐positive children at older ages was due to increasing detection of ASD. Therefore, the reasons for the increase of SCQ mean scores and percentage above cutoff remain uncertain; however, the SCQ is a lifetime measure and score gains with age would be expected. SCQ test–retest results are provided in Appendix S3.
Table 1.
School survey: screening results (N = 9,074)
| N | SCQ total score X (SD) | Screening statusa N (%) | p‐Valueb | ||||
|---|---|---|---|---|---|---|---|
| All | Screen − | Screen + | Negative | Positive | |||
| All subjects | 9,074 | 6.8 (5.3) | 5.7 (3.7) | 19.1 (4.2) | 8,303 (91.5) | 771 (8.5) | |
| Age 5–6 | 1,321 | 6.2 (5.1) | 5.2 (3.6) | 19.8 (4.8) | 1,237 (93.6) | 84 (6.4) | <.001 |
| Age 7–8 | 3,399 | 6.7 (5.3) | 5.6 (3.7) | 19.5 (4.5) | 3,138 (92.3) | 261 (7.7) | |
| Age 9–10 | 2,765 | 6.9 (5.2) | 5.7 (3.7) | 18.4 (3.6) | 2,511 (90.8) | 254 (9.2) | |
| Age 11–12 | 1,589 | 7.4 (5.5) | 6.0 (3.7) | 19.1 (4.3) | 1,417 (89.2) | 172 (10.8) | |
| All boys | 3,716 | 7.2 (5.5) | 5.9 (3.7) | 19.4 (4.3) | 3,358 (90.4) | 358 (9.6) | |
| Age 5–6 | 532 | 6.3 (5.1) | 5.4 (3.7) | 19.8 (4.6) | 498 (93.6) | 34 (6.4) | .01 |
| Age 7–8 | 1,517 | 7.2 (5.5) | 5.9 (3.7) | 19.5 (4.3) | 1,378 (90.8) | 139 (9.2) | |
| Age 9–10 | 1,096 | 7.3 (5.5) | 5.9 (3.8) | 18.7 (3.7) | 975 (89.0) | 121 (11.0) | |
| Age 11–12 | 571 | 7.9 (5.8) | 6.3 (3.7) | 20.3 (4.9) | 507 (88.8) | 64 (11.2) | |
| All girls | 4,960 | 6.6 (5.1) | 5.5 (3.6) | 18.9 (5.1) | 4,572 (92.2) | 388 (7.8) | |
| Age 5–6 | 694 | 6.1 (5.1) | 5.1 (3.5) | 20.1 (5.1) | 649 (93.5) | 45 (6.5) | <.001 |
| Age 7–8 | 1,730 | 6.3 (5.0) | 5.4 (3.6) | 19.4 (4.8) | 1,617 (93.5) | 113 (6.5) | |
| Age 9–10 | 1,560 | 6.6 (5.0) | 5.6 (3.7) | 18.2 (3.5) | 1,437 (92.1) | 123 (7.9) | |
| Age 11–12 | 976 | 7.3 (5.4) | 5.9 (3.7) | 18.5 (3.9) | 869 (89.0) | 107 (11.0) | |
Positive or negative status is defined by a Social Communication Questionnaire (SCQ) total score either ≥ or < 15 (see text).
Comparisons (chi‐square) of the percentages of screen‐negative and screen‐positive children between the 4 age categories.
Diagnostic confirmation phase
Of the 760 screen− children who participated in the second phase, three children (two males, one female) turned out to have ASD that had been already diagnosed. We confirmed these diagnoses by evaluating in‐person one child and used parent interviews and medical record review for the other two. These three children had also been identified by the other case ascertainment approach and were included in the QCC. In the remaining 757 children (mean age: 9.5 years (SD = 1.8); 46.5% male), one child was assessed in‐person and a diagnosis of ASD was definitely ruled out. All other 756 children did not present any behavioral or social characteristic suggestive of ASD. Some children were reported to have other problems including ADHD (N = 4; .5%), developmental problems (e.g. language delay; N = 7; 0.9%), learning difficulties (N = 7; 0.9%), or behavioral problems (N = 1; .1%).
The screen+ sample [45.4% male; mean age: 8.6 years (SD = 2.1)] had a mean SCQ score of 19.1 (SD = 4.5). Among the 163 participants, 14 were found to have ASD [13 males, one female; mean age: 8.0 (SD = 2.1); mean SCQ score: 20.8 (SD = 4.3)]; of these, five cases were newly diagnosed as part of the survey. All but one child had in‐person evaluations. ASD diagnosis in the other child was confirmed with parental interview later corroborated by medical record review. In the remaining 149 children [40.9% male; mean age: 8.6 (SD = 2.1); mean SCQ score: 19.0 (SD = 4.5)], once ASD had been ruled out, the evaluation suggested the following problems: ADHD (N = 9; 6%), behavioral and emotional problems (N = 10; 6.7%), social and adjustment problems (N = 7; 4.7%) and specific learning difficulties (N = 3; 2.0%).
In the combined screen+ and screen− samples, the 17 children identified as having ASD had significantly higher SCQ scores than the reminder sample (19.2 vs. 6.8; p < .001) and were disproportionately males (15 males). There was no statistical difference for mean SCQ scores (20.8 vs. 18.5; NS) or for gender (P = .51) between the five newly diagnosed and the 12 already‐diagnosed children with ASD.
Prevalence estimation
The prevalence of newly diagnosed ASD was 0.32% (0.09%–0.64%). The numbers of screen+ and screen− children participating in diagnostic confirmation were simulated using a binomial distribution with probabilities of 760/8301 and 163/773, respectively, based on observed participation. We drew newly diagnosed cases with probabilities 0.5/760 and 5/163 and calculated the prevalence of new diagnoses in each screened group. We then simulated the screening process, drawing from a binomial with probability 8301/9074 and 773/9074 for screen− and screen+ results, respectively. We multiplied these numbers by our calculated screening‐result‐specific prevalences to find the expected number of cases for our numerator and used the sum of simulated screen+ and screen− as the denominator for the final prevalence estimation.
The prevalence of already‐diagnosed ASD cases from the QCC was 0.82% (95% CI: 0.77–0.87), calculated as 1,094 cases/133,781 children ages 6–11 in the 2015 census.
Summing prevalence of already and newly diagnosed cases, the total prevalence was estimated at 1.14% (0.89%–1.46%)
We tested the impact of potential misclassification. In the QCC, 403 cases aged 6–11 could not be confirmed by the same protocol of full record abstraction and parent interview. If we posit that 10% of these were not actual ASD cases, the prevalence of already‐diagnosed ASD would be 0.79% (0.74%–0.84%). If 20% of the 403 were noncases, the estimated prevalence of already‐diagnosed ASD would be 0.76% (0.71%–0.81%).
Among all children in the QCC, ages 5–12, we found 1,398 cases. Using the census denominator of 175,960 children, we find a prevalence of 0.79% (0.75%–0.83%). Of these cases, 554 could not be included in the full protocol of record abstraction and parental interview. If as many as 20% were non‐ASD cases, the prevalence of already‐diagnosed ASD would be 0.73% (0.69%–0.77%).
Age‐ and gender‐specific estimates appear in Figure 2. We obtained these estimates by calculating the distributions from complete records in the QCC, then applying those same distributions to the simulated QSS + QCC populations and calculating group‐specific prevalence with denominators from 2015 census data.
Figure 2.
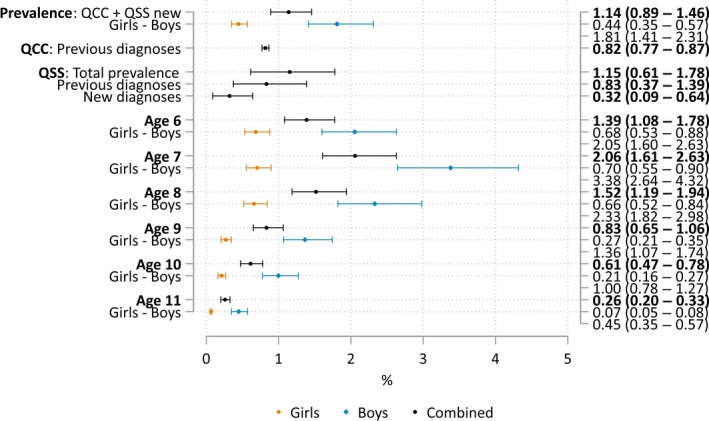
Prevalence estimates (95% confidence intervals), by age and gender
For comparison, we also calculated the prevalence of already‐diagnosed ASD derived only from the school survey. The prevalence of already‐diagnosed ASD from the QSS was 0.83% (0.37%–1.39%). To arrive at this estimate, we used the same simulation described above, but substituted the probabilities 3/760 and 9/163 for already‐diagnosed cases among screen− and screen+ children. Summing new and existing diagnoses, the total prevalence estimated from the QSS only was 1.15% (0.61%–1.78%). Thus, both the total prevalence and the prevalence of already‐diagnosed cases were very close using two independent estimation methods.
Clinical profiles of ASD cases
Clinical data for 844 children (five newly diagnosed from QSS, 839 from QCC) are shown in Tables 2 and 3.
Table 2.
Socio‐demographic and familial characteristics of autism spectrum disorders (ASD) children (N = 844)
| All (N = 844) | Boys (N = 684) | Girls (N = 160) | p‐Value | |
|---|---|---|---|---|
| Children with ASD | ||||
| Mean age, mean (SD) | 7.24 (1.60) | 7.29 (1.62) | 7.03 (1.52) | .056 |
| Age group, No. (%) | ||||
| 5–6 years | 270 (32.0) | 210 (30.7) | 60 (37.5) | .24 |
| 7–8 years | 375 (44.4) | 305 (44.6) | 70 (43.8) | |
| 9–10 years | 142 (16.8) | 119 (17.4) | 23 (14.4) | |
| 11–12 years | 57 (6.8) | 50 (7.3) | 7 (4.4) | |
| Nationality, No. (%) | ||||
| Qatar | 248 (29.5) | 206 (30.2) | 42 (26.4) | .78 |
| Other GCC countries | 19 (2.3) | 15 (2.2) | 4 (2.5) | |
| Other Arabic countries | 351 (41.7) | 280 (41.0) | 71 (44.7) | |
| Other countries | 224 (26.6) | 182 (26.6) | 42 (26.4) | |
| Mother's age at birth | ||||
| Mean age, mean (SD) | 30.3 (5.43) | 30.4 (5.49) | 29.8 (5.18) | .22 |
| Age group, No. (%) | ||||
| <20 | 8 (.9) | 6 (1.1) | 2 (1.5) | .069 |
| 20–24 | 114 (13.5) | 88 (15.7) | 26 (19.0) | |
| 25–29 | 235 (27.8) | 191 (34.0) | 44 (32.1) | |
| 30–34 | 295 (23.1) | 147 (26.2) | 48 (35.0) | |
| 35–39 | 116 (13.7) | 102 (18.1) | 14 (10.2) | |
| ≥40 | 31 (3.7) | 28 (5.0) | 3 (2.2) | |
| Father's age at birth | ||||
| Mean age, mean (SD) | 34.8 (6.48) | 35.1 (6.48) | 33.8 (6.39) | .035 |
| Age group, No. (%) | ||||
| <20 | 37 (4.4) | 25 (4.4) | 12 (8.7) | .13 |
| 20–24 | 134 (15.9) | 108 (19.1) | 26 (18.8) | |
| 25–29 | 223 (26.4) | 172 (30.5) | 51 (37.0) | |
| 30–34 | 164 (19.4) | 136 (24.1) | 28 (20.3) | |
| 35–39 | 90 (10.7) | 78 (13.8) | 12 (8.7) | |
| ≥40 | 54 (6.4) | 45 (8.0) | 9 (6.5) | |
| Maternal education level, No. (%) | ||||
| Primary school | 30 (3.6) | 23 (3.4) | 7 (4.6) | .54 |
| Secondary school | 152 (18.0) | 129 (19.1) | 23 (15.2) | |
| College | 489 (57.9) | 394 (58.5) | 95 (62.9) | |
| Master or PhD | 154 (18.2) | 128 (19.0) | 26 (17.2) | |
Table 3.
Obstetric, birth, developmental history, and current clinical characteristics of autism spectrum disorders children (N = 844)
| All (N = 844) | Boys (N = 684) | Girls (N = 160) | p‐Value | |
|---|---|---|---|---|
| Pregnancy duration in weeks, mean (SD) | 39.5 (1.48) | 39.6 (1.46) | 39.4 (1.52) | .23 |
| Prematurity (<37 weeks), No. (%) | 41 (5.1) | 29 (4.4) | 12 (7.7) | .095 |
| Obstetric complications | ||||
| Gestational diabetes, No. (%) | 72 (8.9) | 56 (8.5) | 16 (10.3) | .48 |
| Gestational hypertension, No. (%) | 26 (3.2) | 16 (2.4) | 10 (6.5) | .011 |
| Any gestational complication, No. (%) | 111 (13.7) | 84 (12.8) | 27 (17.4) | .13 |
| Birthweight, mean (SD) | 3,149 (565) | 3,174 (565) | 3,045 (556) | .017 |
| Birthweight <2,500 g, No. (%) | 56 (8.0) | 42 (7.4) | 14 (10.4) | .24 |
| C‐section delivery, No. (%) | 239 (29.5) | 192 (29.3) | 47 (30.5) | .76 |
| Postnatal complications | ||||
| Hypoxia, No. (%) | 45 (5.5) | 35 (5.3) | 10 (6.4) | .60 |
| Jaundice, No. (%) | 68 (8.4) | 58 (8.9) | 10 (6.4) | .32 |
| Any complication, No. (%) | 124 (15.1) | 101 (15.3) | 23 (14.4) | .77 |
| Motor delay | ||||
| Sitting ≥8 months, No. (%) | 60 (7.6) | 51 (7.9) | 9 (6.0) | .42 |
| Walking ≥18 months, No. (%) | 66 (8.4) | 52 (8.1) | 14 (9.5) | .57 |
| Language delay | ||||
| First words ≥24 months, No. (%) | 572 (75.1) | 461 (74.1) | 111 (79.3) | .20 |
| First phrases ≥33 months, No. (%) | 687 (91.4) | 552 (90.9) | 135 (93.1) | .40 |
| Developmental regression/loss of skills, No. (%) | 156 (19.4) | 133 (20.3) | 23 (15.3) | .17 |
| First parental concerns in months, mean (SD) | 24.5 (7.7) | 24.5 (7.7) | 24.5 (7.8) | .92 |
| Age at diagnosis in months, mean (SD) | 42.8 (15.1) | 43.0 (15.3) | 42.2 (14.3) | .59 |
| Delay between first parental concerns and age at diagnosis in months, mean (SD) | 18.4 (14.1) | 18.5 (SD = 14.3) | 17.9 (13.1) | .67 |
| Current clinical characteristics | ||||
| Language level | ||||
| Receptive language | ||||
| Poor to limited comprehension, No. (%) | 594 (71.0) | 480 (70.4) | 114 (73.5) | .43 |
| Expressive language | ||||
| Limited language, or nonverbal | 164 (19.4) | 122 (17.8) | 42 (26.3) | .015 |
| School functioning | ||||
| Reduced peer interactions, No. (%) | 117 (14.0) | 89 (13.0) | 28 (18.1) | .10 |
| Academic performance difficulties, No. (%) | 56 (7.0) | 45 (6.9) | 11 (7.4) | .83 |
| Has a learning disability, No. (%) | 687 (82.1) | 560 (82.1) | 127 (81.9) | .96 |
| Requires behavioral support at school, No. (%) | 172 (20.5) | 141 (20.7) | 31 (19.9) | .82 |
| Behavioral, emotional adjustment | ||||
| ADHD symptoms, No. (%) | 255 (30.2) | 213 (31.1) | 42 (26.3) | .22 |
| Aggression symptoms, No. (%) | 47 (5.6) | 37 (5.4) | 10 (6.3) | .68 |
| Anxiety symptoms, No. (%) | 93 (11.0) | 75 (11.0) | 18 (11.3) | .92 |
| Behavioral/emotional problem | ||||
| Any problem, No. (%) | 308 (36.5) | 256 (37.4) | 52 (32.5) | .24 |
| Two or more problems, No. (%) | 73 (8.6) | 57 (8.3) | 16 (10.0) | .50 |
| Sleeping problems, No. (%) | 74 (8.8) | 59 (8.6) | 15 (9.4) | .76 |
| Current interventions | ||||
| Psychotropic medication, No. (%) | 53 (6.5) | 41 (6.0) | 12 (7.5) | .48 |
| Speech/language therapy, No. (%) | 35 (4.1) | 29 (4.2) | 6 (3.8) | .78 |
| Behavior therapy, No. (%) | 38 (4.5) | 32 (4.7) | 6 (3.8) | .61 |
| Occupational therapy, No. (%) | 47 (5.6) | 41 (6.0) | 6 (3.8) | .26 |
| Special Need school, No. (%) | 108 (12.8) | 86 (12.6) | 22 (13.8) | .69 |
| Individualized school intervention, No. (%) | 102 (12.1) | 88 (12.9) | 14 (8.8) | .15 |
The overall male:female ratio was 4.3:1, and the mean age was 7.24 years. A third of the sample originated from GCC countries. Children from non‐GCC countries came from Egypt (15.5%), Sudan (13.0%), India (11.3%), the Philippines (11.0%), Jordan (7.5%), Yemen (7.3%), Syria (4.9%), Pakistan (3.7%), Lebanon (3.5%), Palestine (3.3%), and the reminder from 29 other countries. Overall, there were 73.4% of children from Arabic countries and 26.6% from non‐Arabic countries. Of note, the proportion of Qatari children with ASD (29.5%) was similar to that (31.8%) of Qatari children in the whole school population age 5–12. Consanguinity was reported by 160 (19.9%) parents in the whole sample; however, when restricted to families from GCC countries, consanguinity was endorsed by 116 of 249 families (46.6%) and by 109 of 233 (46.8%) Qatari families.
The average number of full siblings (not counting the index child with ASD) was 2.1 (SD = 1.7) and that of half‐siblings was 0.15 (SD = 0.94). After excluding children without sibling or with missing data, 42 out of 659 (6.4%) parents reported to have another child with ASD either in full (N = 38; 5.8%) or half‐siblings (N = 4; 0.6%), or in a more distant relative in 47 families (7.1%). Combined together, ASD recurrence among any relative was reported in 11.8% (78/659) families with available data. Exact sibling recurrence risks could not be calculated in the absence of detailed information about sibship size and order. Similar levels of consanguinity were found in families with or without sibling ASD recurrence (26.2% vs. 22.1%; χ2 = .38, df = 1; NS) or any relative ASD recurrence (27.3% vs. 21.7%; χ2 = 1.22, df = 1; NS).
The mean duration of gestation was 39.5 weeks (SD = 1.47); 5.1% pregnancies had a duration <37 weeks. Pregnancy was uncomplicated in 700 (86.3%) of 811 cases with information. In the remaining 111 pregnancies, medical complications reported were diabetes (N = 72; 8.9%), high blood pressure (N = 26; 3.2%), and other miscellaneous complications (N = 22; 2.7%). Delivery was by C‐section in 239 (29.5%) children; mean birthweight (BW) was 3,149 grams (SD = 565) with 8% BW under 2,500 g. Complications in the postnatal period were hypoxia (N = 45; 5.5%), jaundice (N = 68; 8.4%), and other complications (N = 25; 3.1%); 15 children (1.8%) experienced two or more complications. Gestational diabetes increased the odds of gestational hypertension (11.1% vs. 2.4%; OR = 5.0; 95% CI: 2.1–12.0), delivery by C‐section (41.7% vs. 28.2%; OR = 1.82; 95% CI: 1.11–2.98), prematurity (13.9% vs. 4.2%; OR = 3.68; 95% CI: 1.72–7.87), low BW (24.6% vs. 6.4%; OR = 4.79; 95% CI: 2.47–9.29), neonatal hypoxia (19.4% vs. 4.2%; OR = 5.50; 95% CI: 2.77–10.93) and of any postnatal complication (29.2% vs. 14.0%; OR = 2.54; 95% CI: 1.47–4.40). Mothers with gestational diabetes were slightly but not significantly older (mean age difference: 1.15 years; p = .095) than mothers without diabetes.
Both breastfeeding and formula were used in 87.9% of children, with 6.5% on exclusive breastfeeding. A total of 89 children (11%) required hospitalization during infancy, most often for seizures (N = 18 (2.2%); 15 (1.85%) for febrile seizures). For 19 children (2.3%), parents reported some head trauma with eight children (1%) requiring admission. Vaccinations were up to date in 809 children (96.8%).
Motor milestones were delayed for sitting in 60 children (7.6%) and for walking in 66 children (8.4%). The median age was 24 months for use of first words (interquartile range: 23–30 months) and 48 months for phrases (interquartile range: 41–60 months); 90 children (12.0%) had no phrase speech at the time of the survey. The frequency of language delay was 75.1% (572/762) for first words and 91.4% (687/752) for phrase speech. Parents reported developmental regression or a loss of skills in 156 of 806 children (19.4%). The skill most commonly lost was speech (N = 135; 86.5%) with 17 children (10.9%) having lost at least two skills (predominantly language and social skills).
The median age for first parental concerns was 24 months (range: 4–60 months), with 77.3% being concerned by age 24 months (97.4% by age 36 months). The mean age at diagnosis was 42.8 months (median: 36; range: 12–132). About half the sample (54.0%) was diagnosed by age 36 months, 84.6% by age 48 months and 93.2% by age 60 months. The mean interval between first parental concerns and age at diagnosis was 18.4 months (SD = 14.1; median: 12 months) and was significantly correlated with both age at diagnosis (Spearman's rho = .77; p < .001) and age at first parental concerns (Spearman's rho = −.25; p < .001).
All children were diagnosed with ASD by multidisciplinary team evaluations with experienced clinicians. One standardized diagnostic measure was used in the majority of cases: the ADOS (Lord et al., 2002) (N = 600), the ADI‐R (Rutter, LeCouteur et al., 2003; Rutter, Bailey, et al., 2003) (N = 101), and the CARS (N = 114). Protocols of diagnostic measures and cognitive tests were often filed separately and could not generally be abstracted. Of 314 children with available information, Fragile X disorder was found in two children (one male, one female), Rett syndrome in three children (all females), Down syndrome in two males, Joubert syndrome in one male, Goldenhar syndrome in one male, blindness in three males, and hearing impairment in one male. There was no report of congenital rubella, tuberous sclerosis, or neurofibromatosis. Epilepsy was reported by history in 102 children (12.1%; boys: 12.0% and girls: 12.5%; NS).
Current clinical, educational and behavioral characteristics are presented in Table 3. Over two‐thirds of the sample had limitations in receptive language, whereas a fifth had substantial impairments in expressive language. The majority of children had difficulty to learn at school, and about 15%–20% also appeared socially isolated or required behavioral support in the classroom. Of the comorbid behavioral problems, ADHD symptoms were the most common, affecting almost a third of the sample. Anxiety, ADHD and aggression were strongly associated (p < .001) with 36.5% of children showing at least one emotional or behavioral syndrome, and 8.6% showing at least two of them. Use of psychotropic medications was limited to 53 children (6.5%) and included risperidone (N = 33), anticonvulsants (N = 13) and stimulants or other anti‐ADHD drugs (N = 7). The odds of psychotropic medication use were raised significantly with the presence of ADHD (11.4% vs. 4.1%; OR = 2.67; 95% CI: 1.48–4.82), marginally with aggression (17.0% vs. 5.7%; OR = 2.26; 95% CI: 0.94–5.40), but not with anxiety (9.7% vs. 5.9%; OR = 1.11; 95% CI: 0.50–2.47). Other individual therapies were currently delivered in a small (≈5%) proportion of the sample, whereas school‐based interventions were more than twice as frequent. Other current interventions occurring in fewer than 30 children (not shown in Table 3) were special diet (N = 18; 2.1%), vitamin supplements (N = 16; 1.9%), and hyperbaric oxygen therapy (N = 6; 0.7%). The frequency of interventions was much higher when past treatments were counted, with most children having received speech and language therapy (96.5%), behavioral intervention (97.0%), and occupational therapy (79.4%). With the exception of special diet (17.9% of subjects), other interventions remained at lifetime low levels (medications: 10.3%; vitamin supplements: 1.9%; hyperbaric oxygen therapy: 1.1%).
Sex differences
Girls had significantly lower birthweights (Table 3), a difference that persisted after exclusion of children of low birthweights (boys: 3,269; girls: 3,173 g; p = .037). Girls were significantly more impaired than boys on expressive language functioning and were more socially isolated from peers although this was only a trend (Table 3). Otherwise, age at first parental concerns and diagnosis, patterns of behavioral/emotional problems, and their comorbidity, as well as service use, were similar across genders.
ASD severity
Binary logistic regression models evaluated associations between low versus high severity as measured by the CSS and 28 independent predictors (see Table S2). Child gender, paternal and maternal age, pregnancy duration, prematurity, delivery by C‐section, language delay (first words), age at diagnosis, and delay between first parental concerns and diagnosis were not associated with severity using a cutoff of p < .15 for variable selection. To prevent multicollinearity, we dropped birthweight (continuous), any obstetric complication, and any postnatal complication. The 15 remaining variables (five demographic/family, five obstetric/perinatal variables, five developmental/clinical) were entered into a multivariate logistic regression using the forward selection procedure. The final model had good fit and contained four variables: developmental regression (OR = 2.51; 95% CI: 1.69–3.74) entered the model first, followed by motor delay (OR = 2.83; 95% CI: 1.59–5.03), gestational diabetes (OR = 2.46; 95% CI: 1.42–4.25), and consanguinity (OR = 1.68; 95% CI: 1.10–2.57). The same final model was obtained using a backward selection procedure (see full model in Table S3).
Consanguinity and phenotypic expression
In 249 families (112 consanguineous) from the Gulf countries, the prevalence of first‐degree consanguinity was 45.0% (95% CI: 0.41–0.53%). Consanguinity was not associated with maternal education, child gender, or ASD recurrence in siblings and relatives (all P's > .50). We further tested for associations between first‐degree consanguinity and 37 clinical variables included in Table 3. Gestational diabetes and the occurrence of any obstetric complication were more frequent in the consanguineous group (gestational diabetes: 17.9% vs. 8.8%; p = .036; any gestational complication: 23.6% vs. 11.0%; p = .009). Compared to children from nonconsanguineous parents, children from consanguineous parents had lower mean birthweights (2,972 vs. 3,235; p = .001) and higher frequency of low BW (18.2% vs. 2.7%; p < .001). All other 33 comparisons yielded statistically nonsignificant results.
Discussion
Our study incorporated recent improvements in autism survey methodology, specifically a general school population screening to identify undiagnosed ASD. Participation in the school survey was acceptable albeit low in the first screening phase. The screening questionnaire had good specificity only identifying a small (<10%) manageable proportion of screen+ children. We succeeded in evaluating a high number of children in both the screen− and screen+ groups permitting precise final case status determination in almost a thousand participants. Our reliance on a flexible combination of telephone interviews and in‐person clinical evaluations enhanced families’ participation while optimizing our resources. Identification of newly diagnosed children allowed us to reduce the bias in estimating prevalence that inevitably occurs when only already‐diagnosed children are ascertained.
Our estimate of 1.14% is broadly in line with recent studies (Arora et al., 2018; Baio et al., 2018; Diallo et al., 2018) and reviews (Elsabbagh et al., 2012; Hill et al., 2014; Meyers et al., 2018). Compared to previous regional surveys (see Table S1), our more comprehensive methodology resulted in a much higher estimate. Other factors may have contributed to the difference; Qatar has a well‐funded medical/educational system allowing access to services to all its residents, national policies for individuals with disabilities exist, well‐trained teams with autism expertise were available, public and professional ASD awareness recently increased, and funding was readily available.
Our prevalence had two different components. The school survey component (0.32%) uniquely due to newly diagnosed children accounted for 28.1% (0.32/1.14) of the overall prevalence. This compares to school survey prevalences of 0.50% (57.5% of overall prevalence) in the Mexico study (Fombonne et al., 2016) and 1.89% (71.6% of overall prevalence) in the South Korea study (Kim et al., 2011). These remarkable disparities may reflect differences in study methods such as variability in participation rates and sampling fractions, in screening tools and diagnostic methods and in weighted statistical analyses. Alternatively, they may reflect true population differences with respect to autism awareness, detection and identification of ASD, access to medical diagnoses, and extent of supportive school services. However, as the school component proportion increases steadily with overall prevalence, it is unlikely that lack of awareness and pervasive underdiagnosing are contributory since a reverse pattern would otherwise be expected, with relatively more undiagnosed cases in lower prevalence studies. Of note, all studies showed that prevalence would be underestimated if school surveys had not been performed to identify children without an existing diagnosis. The magnitude of the corresponding bias is difficult to evaluate. In our more conservative study, the downward bias in prevalence would be −28%. These findings have implications for designing future surveys and interpreting existing surveillance data.
Our estimate for 8‐year‐olds (the age group surveyed by CDC) was 1.53% (Figure 2), which is similar to the three most recent CDC prevalence of 1.49%, 1.46%, and 1.69% (Baio et al., 2018; Christensen et al., 2016; ADDM, 2014). However, it incorporates a school survey component, a feature lacking in the CDC methodology. Remarkably, 20% of cases in CDC surveys were not previously diagnosed either. New diagnoses were derived from record reviews but not confirmed with direct evaluations, resulting in diagnostic validity concerns (Fombonne, 2018; Mandell & Lecavalier, 2014). Even when estimates converge, comparisons between studies should be made cautiously given numerous method differences that may confound comparisons in both directions.
A unique study feature was to generate two independent estimates for the prevalence of already‐diagnosed cases; one through a head count of clinical/educational sites, the other derived from a two‐phase survey of the general school population. Although expected, the convergence of the two estimates (0.83% and 0.82%) was surprisingly close. To the best of our knowledge, this is the first survey that provided such an internal replication of its estimation procedures. Future surveys would benefit to build in their design similar replication approaches.
The performances of the SCQ (Rutter, LeCouteur et al., 2003; Rutter, Bailey et al., 2003) as a screening tool proved very adequate. The negative predictive value was very high (757/760 = 99.6%), the specificity was excellent (757/906 = 83.6%), the test–retest reliability was satisfactory, and the positive predictive value (PPV) was acceptable for a relatively rare disorder (14/163 = 8.6%). Previous studies reporting similar or better SCQ performance were usually conducted on clinical samples of preschoolers, a context very different from our population screening at school age (Charman et al., 2016). The somewhat low PPV required testing a relatively high number of children to eliminate false positives, a common problem in ASD surveys with all available screening instruments. To achieve higher specificity and PPV, it may be necessary to employ multisteps screening approaches rather than to rely on one‐time administration of a questionnaire. Selecting persistently high scorers over several administrations of the same or different screening instruments over short time intervals, or even combining questionnaire‐based informant screening followed by rapid clinician‐driven group procedures to rule out ASD (Zhou et al., unpublished data) may improve the efficiency of population screening.
As expected, prevalence was four times as high in boys than in girls, and there was a trend toward more severe outcome among girls. Age‐specific prevalence estimates were variable ranging from 0.26 among 11‐year‐olds to 2.06 among 7‐year‐olds. The decline in rates from age 8 to 11 is not consistent with the natural history of ASD. Because age‐specific estimates were derived solely from the QCC observed case distribution, the age pattern has no relationship with the QSS screening results and is unrelated to the unexpected association of SCQ screening status and age. Similarly, intriguing results were reported in a pilot CDC study (Yeargin‐Allsopp et al., 2003) that contributed to choosing age 8 as the most appropriate age for surveillance. Our age pattern is consistent with increasing awareness and improved detection and diagnosis having occurred recently in Qatar. Likewise, training of ASD teams at our clinical sites occurred in the late 2000s, and it is therefore possible that older children, when evaluated as preschoolers, may have been underdiagnosed and misclassified. For all these reasons, rates in children of age 9–11 should be considered as underestimates.
Clinical profiles of ASD in this study were very similar to those from other population surveys. There was a typical preponderance of males with ASD; language delay was a prominent developmental feature and, consistent with other studies (ADDM, 2014; [Link]; Zhou et al., unpublished data), regression often involving language skills occurred in a fifth of subjects. Also consistent with the literature (Di Giacomo & Fombonne, 1998; Shattuck et al., 2009), parents became concerned around 2 years of age, yet a formal diagnosis was established only 18 months later outlining the long wait experienced by parents. Nevertheless, an average age at diagnosis around 3.5 years compares well with data from other countries (Shattuck et al., 2009). At the time of the survey, a substantial proportion of children showed persisting social and communication deficits, with 12% having no phrase speech. Unfortunately, IQ data were not available on most children, which was a strong limitation of this study. In line with other studies (Hartman, Geurts, Franke, Buitelaar, & Rommelse, 2016; Simonoff et al., 2008), comorbid behavioral problems were reported in up to a third of subjects, with ADHD being the leading associated problems. Use of speech/language, occupational, and behavior therapy were at low levels in this school‐age sample; however, access to these evidence‐based therapies in early childhood was at much higher rates. It was satisfying that vaccine uptake was high and use of non‐evidence‐based therapies (such as hyperbaric oxygen treatment and vitamin supplements) remained infrequent.
Our study provided a unique opportunity to examine consanguinity in a large ASD sample. Consanguinity in the sample of Qatar/GCC families was comparable to other published rates for the Qatari and GCC populations (e.g. 54% or 46.7%) (Bener & Alali, 2006; Bener et al., 2013). From these comparisons, it does not appear that consanguinity is a risk factor for ASD although controlled epidemiological studies would be preferred to test this link. However, and supporting a lack of relationship between consanguinity and autism risk, we found no relationship between consanguinity and familial recurrence of ASD. By contrast, consanguinity predicted ASD severity after controlling for the effect of maternal health and developmental indicators. This original finding suggests that background genetic factors and increased homozygosity may modulate ASD phenotypic expression beyond and above the effect of primary causal, genetic or otherwise, risk factors. This novel finding requires replication, but it is worth noting that increased disease severity in offspring of consanguineous parents has already been reported, for example for congenital heart defects associated with Down syndrome (Bittles & Black, 2010).
Evidence from other studies or meta‐analyses suggests that gestational diabetes, gestational hypertension, C‐section, low BW, and hypoxia are risk factors for ASD (Curran et al., 2018; Flygare Wallén, Ljunggren, Carlsson, Pettersson, & Wändell, 2018; Hisle‐Gorman et al., 2018; Maher et al., 2018; Xu, Jing, Bowers, Liu, & Bao, 2014). Compared to Qatar studies (Bener, Saleh, & Al‐Hamaq, 2011; Bener et al., 2013), our observed rates were not raised; however, in the absence of non‐ASD controls, interpretation must be guarded. Our data suggested that gestational diabetes was a significant predictor of other obstetric and perinatal complications, as shown in other studies in Qatar (Bener et al., 2011) and elsewhere. Interestingly, gestational diabetes was the only obstetric and perinatal variable that remained a significant predictor of severity in our multivariate analysis; five other variables (prematurity, hypertension, low birthweight, hypoxia, and jaundice) that predicted severity in unadjusted binary models lost significance when gestational diabetes was adjusted for. Unfortunately, further analyses of gestational diabetes were limited as we did not have data on potential confounders such as maternal obesity, prepregnancy body mass index, gestational weight gain, as well as interpregnancy interval and parity.
Besides gestational diabetes and consanguinity, severity was independently associated with two developmental clinical features. First, delay in walking was reported at a relatively high frequency in our sample and likely acted as a proxy variable for cognitive delay. Second, developmental regression independently predicted severity once the effect of other predictors was adjusted for, a finding consistent with most published studies (Bernabei, Cerquiglini, Cortesi, & D'Ardia, 2007; Meilleur & Fombonne, 2009; Richler et al., 2006; Zachor & Ben‐Itzchak, 2016). Loss of skills was reported in about a fifth of children with ASD, a proportion which is consistent with that reported in previous clinical (Meilleur & Fombonne, 2009) and epidemiological samples (Thurm, Powell, Neul, Wagner, & Zwaigenbaum, 2018). Since regression is highly specific to ASD (Pickles et al., 2009), occurs in the second year of life, and predicts later severity, improved recognition of this early marker of ASD could help direct early intervention to vulnerable children and ameliorate ASD developmental trajectories.
Our investigation has several strengths including comprehensive case identification at the population level, the addition of a general school survey to identify undiagnosed children, reliance on both health and education sources to ascertain cases, use of autism‐specific screening instruments and diagnostic tools, a large sample size, a high proportion of screened children assessed for caseness determination (especially that achieved for screen‐negative participants), statistical analyses that increased confidence in the estimates, and novel findings on consanguinity. Some limitations are also noted. First, like in other school surveys (Fombonne et al., 2016; Kim et al., 2011), parent participation was low and no data were available to evaluate whether or not participation was differentially associated with caseness. As a result, we hypothesized, but could not demonstrate, that nonparticipation was independent of the presence/absence of ASD in the family. As a consequence, the school survey prevalence component could have been overestimated. Second, our plan to abstract clinical information on all prevalent cases could not be fully executed due to limited resources. Yet, there was evidence that abstracted and unabstracted cases were comparable and sensitivity analyses showed limited impact of potential misclassification on prevalence estimates. Third, data available from record reviews supplemented by parental reports did not include scores of standardized tests, both diagnostic and cognitive, or results of medical tests.
There are several take‐home messages. First, our results support adding population screening to increase sensitivity of case ascertainment in epidemiological studies. Second, considering our 1.14% prevalence and GCC population demographics, we estimated that, in GCC countries, there are about 50,500 children under age 5 and 137,000 subjects in the 5–19 age range with ASD (Qatar only: 1,575 and 5,025, respectively). These figures point at the need both for early detection, diagnosis and early intervention programs for preschoolers and for education, intervention and vocational training programs required at later ages, in order to reduce individual and societal costs associated to autism across the life span (Buescher, Cidav, Knapp, & Mandell, 2014). Third, we validated tools and a survey methodology for Qatar that can be effectively deployed in other countries in the region. Fourth, the results of this first survey should be regarded as a baseline against which future monitoring of ASD incidence and prevalence in Qatar can be calibrated. Finally, children of our prevalence pool and their families have been already invited to participate in a national registry that will facilitate future research on individuals with ASD of all ages.
Key points.
Qatar first ASD prevalence estimate of 1.14% is consistent with that of other populations.
The methodology of this survey confirms that comprehensive case ascertainment requires systematic screening of the general child population in order to identify yet‐undiagnosed children with ASD.
Compared to the general population, consanguinity between parents of children with ASD was not raised, and recurrence of ASD within families was not associated with consanguinity. However, consanguinity predicted increased severity of ASD.
We estimated that 187,000 youths under age 20 have ASD in Arabic countries within the Gulf region; these figures have implications for planning improved health and education services.
Supporting information
Appendix S1. Qatar Clinical Centers (QCC) sites.
Appendix S2. Other study features.
Appendix S3. Test‐retest reliability.
Table S1. Prior surveys of ASD in Arabic countries.
Table S2. Predictors of severity: results of univariate logistic regression models (N = 844).
Table S3. Predictors of ASD severity: final multivariate logistic model (N = 737).
Acknowledgements
The study was supported by the Qatar National Research Fund. The sponsor had no role in the design and conduct of the study, or the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript, and in the decision to submit the manuscript for publication.
The authors thank all the independent and private schools that have participated in the SCQ screening phase. The authors thank the Ministry of Public Health and the Ministry of Education for providing their team with the data required throughout the study. The authors would like to express our gratitude to the psychological services team at the Shafallah Center for Children with Disabilities, as well as Dr. Irshad Shafeullah and Dr. Zakariah Al‐Sayed for their support in patient recruitment. The authors also thank our colleagues from QBRI: Dr. Hatem Al‐Shanti for his assistance in patient phenotyping, and Mr. Yasser Al‐Sarraj and Ms. Hamda AlMutawwa for their support with patient recruitment. The authors thank Dr. Hanaa Massoud for assisting with patient recruitment through the clinic at the Child Development Center in Rumailah Hospital. The authors would also like to express their appreciation to the QBRI administration team for their assistance with planning and logistics pertaining to research‐related training sessions and research collaborator visits. Additionally, the authors would like to thank all of the special needs centers and clinics which collaborated with us to provide data needed for the high probability cases; Shafallah Center for Children with Disabilities, Hamad Medical Corporation, Child Development Center – Rumailah Hospital, Child Development Center‐Private, Renad Academy, Al‐Tamakun school, Step by Step Center, Qatar Institute for Speech and Hearing, and Hope Center. The authors also thank the HBKU Sponsored Research Office for the support provided throughout the research funding period. K.R. and the OHSU Biostatistics & Design Program was partially supported by the Oregon Clinical and Translational Research Institute (OCTRI) through OHSU Clinical & Translational Science Awards (CTSA UL1TR0002369) National Consortium. The authors are immensely grateful to the families and their children for their time and participation in any of the phases of the research.
F.A. and E.F. designed the research plan. F.A., M.A., and E.F. applied for funding. F.A., H.A., S.E., I.G., M.T., M.A., M.K., N.A.A., M.A., A.H.S., and L.D. organized the data collection. F.A., H.A., S.E., and I.G. completed data entry and cleaning, and performed initial data analyses with M.A.. E.F. performed data and statistical analyses. K.R. provided biostatistical advice. E.F., F.A., and I.G. wrote the manuscript. All authors reviewed and approved the manuscript. The authors have declared that they have no competing or potential conflicts of interest.
Conflict of interest statement: No conflicts declared.
The copyright line for this article was changed on 23 May 2019 after original online publication.
References
- Aldosari, M. , Fombonne, E. , Aldhalaan, H. , Ouda, M. , Elhag, S. , Alshammari, H. , … & Alshaban, F. (2019). Validation of the Arabic version of the Social Communication Questionnaire. Autism, 23, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaban, F. (2018). Qatar and autism. Encyclopedia of autism spectrum disorders. New York: Springer Science. [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed) DSM‐5. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- American Psychiatric Association (APA) (2000). Diagnostic and statistical manual of mental disorders: DSM‐IV‐TR (4th edition, text revision). Washington, DC: American Psychiatric Association. [Google Scholar]
- Arora, N.K. , Nair, M.K.C. , Gulati, S. , Deshmukh, V. , Mohapatra, A. , Mishra, D. , … & Vajaratkar, V. (2018). Neurodevelopmental disorders in children aged 2–9 years: Population‐based burden estimates across five regions in India. Journal of the Public Library of Science, 15, e1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring (ADDM) Network Surveillance Year 2010 Principal Investigators . (2014). Prevalence of autism spectrum disorder among children aged eight years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report (MMWR) Surveillance Summary, 63(No. SS‐2): 1–21. [PubMed] [Google Scholar]
- Baio, J. , Wiggins, L. , Christensen, D.L. , Maenner, M.J. , Daniels, J. , Warren, Z. , … & Dowling, N.F. (2018). Prevalence of autism spectrum disorder among children aged 8 years ‐ autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. Morbidity and Mortality Weekly Report (MMWR) Surveillance Summary, 67, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bener, A. , & Alali, K.A. (2006). Consanguineous marriage in a newly developed country: The Qatari population. Journal of Biosocial Science, 38, 239–246. [DOI] [PubMed] [Google Scholar]
- Bener, A. , Al‐Nufal, M. , Vachhani, P.J. , Ali, A.I. , Samson, N. , & Saleh, N.M. (2013). Maternal complications and neonatal outcome in Arab women of a fast developing country. Journal of Family and Community Medicine, 20, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bener, A. , Saleh, N.M. , & Al‐Hamaq, A. (2011). Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast‐developing community: Global comparisons. International Journal of Women's Health, 3, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabei, P. , Cerquiglini, A. , Cortesi, F. , & D'Ardia, C. (2007). Regression versus no regression in the autistic disorder: Developmental trajectories. Journal of Autism and Developmental Disorders, 37, 580–588. [DOI] [PubMed] [Google Scholar]
- Berument, S.K. , Rutter, M. , Lord, C. , Pickles, A. , & Bailey, A. (1999). Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry, 175, 444–451. [DOI] [PubMed] [Google Scholar]
- Bittles, A.H. , & Black, M.L. (2010). The impact of consanguinity on neonatal and infant health. Early Human Development, 86, 737–741. [DOI] [PubMed] [Google Scholar]
- Buescher, A.V. , Cidav, Z. , Knapp, M. , & Mandell, D.S. (2014). Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatrics, 168, 721–728. [DOI] [PubMed] [Google Scholar]
- Charman, T. , Baird, G. , Simonoff, E. , Chandler, S. , Davison‐Jenkins, A. , Sharma, A. , … & Pickles, A. (2016). Testing two screening instruments for autism spectrum disorder in UK community child health services. Developmental Medicine and Child Neurology, 58, 369–375. [DOI] [PubMed] [Google Scholar]
- Christensen, D.L. , Baio, J. , Van Naarden Braun, K. , Bilder, D. , Charles, J. , Yeargin‐Allsopp, M. , … & Centers for Disease Control and Prevention (CDC) (2016).Prevalence and characteristics of autism spectrum disorder among children aged 8 years–Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morbidity and Mortality Weekly Report (MMWR) Surveillance Summary, 65: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran, E.A. , O'Keeffe, G.W. , Looney, A.M. , Moloney, G. , Hegarty, S.V. , Murray, D.M. , … & Kenny, L.C. (2018). Exposure to hypertensive disorders of pregnancy increases the risk of autism spectrum disorder in affected offspring. Journal of Molecular Neurobiology, 55, 5557–5564. [DOI] [PubMed] [Google Scholar]
- Di Giacomo, A. , & Fombonne, E. (1998). Parental recognition of developmental abnormalities in autism. European Child Adolescent Psychiatry, 7, 131–136. [DOI] [PubMed] [Google Scholar]
- Diallo, F.B. , Fombonne, E. , Kisely, S. , Rochette, L. , Vasiliadis, H.M. , Vanasse, A. , … & Lesage, A. (2018). Prevalence and correlates of autism spectrum disorders in Quebec. Canadian Journal of Psychiatry, 63, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves, L.C. , Wingert, H.D. , Ho, H.H. , & Mickelson, E.C.R. (2006). Screening for autism spectrum disorders with the social communication questionnaire. Journal of Developmental and Behavioral Pediatrics, 27, 95–103. [DOI] [PubMed] [Google Scholar]
- Elsabbagh, M. , Divan, G. , Koh, Y.J. , Shin Kim, Y.S. , Kauchali, S. , Marcín, C. , … & Fombonne, E. (2012). Global prevalence of Autism and other pervasive developmental disorders. Autism Research, 5, 160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flygare Wallén, E. , Ljunggren, G. , Carlsson, A.C. , Pettersson, D. , & Wändell, P. (2018). High prevalence of diabetes mellitus, hypertension and obesity among persons with a recorded diagnosis of intellectual disability or autism spectrum disorder. Journal of Intellectual Disability Research, 62, 269–280. [DOI] [PubMed] [Google Scholar]
- Fombonne, E. (2009). Epidemiology of pervasive developmental disorders. Pediatric Research, 65, 591–598. [DOI] [PubMed] [Google Scholar]
- Fombonne, E. (2018). The rising prevalence of autism. Journal of Child Psychology and Psychiatry, 59, 717–720. [DOI] [PubMed] [Google Scholar]
- Fombonne, E. , Marcin, C. , Manero, A.C. , Bruno, R. , Diaz, C. , Villalobos, M. , … & Nealy, B. (2016). Prevalence of autism spectrum disorders in Guanajuato, Mexico: The Leon Survey. Journal of Autism and Developmental Disorders, 46, 1669–1685. [DOI] [PubMed] [Google Scholar]
- Hamad Medical Corporation . (n.d.). Autism program. Available from: https://www.hamad.qa/ [last accessed 1 September 2018].
- Hartman, C.A. , Geurts, H.M. , Franke, B. , Buitelaar, J.K. , & Rommelse, N.N. (2016). Changing ASD/ADHD symptom co‐occurrence across the lifespan with adolescence as crucial time window: Illustrating the need to go beyond childhood. Journal of Neuropsychopharmacology, 71, 529–541. [DOI] [PubMed] [Google Scholar]
- Hill, A.P. , Zuckerman, K. , & Fombonne, E. (2014). Epidemiology of autism spectrum disorders In Volkmar F.R., Rogers S.J., Paul R., & Pelphrey K.A. (Eds.), Handbook of autism and pervasive developmental disorders (4th ed.). Diagnosis, development, and brain mechanisms, 1 (pp. 57–96). New York: Wiley & Sons. [Google Scholar]
- Hisle‐Gorman, E. , Susi, A. , Stokes, T. , Gorman, G. , Erdie‐Lalena, C. , & Nylund, C.M. (2018). Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Journal of Pediatric Research, 84, 190–198. [DOI] [PubMed] [Google Scholar]
- Kim, Y.S. , Leventhal, B.L. , Koh, Y.J. , Fombonne, E. , Laska, E. , Lim, E.C. , … & Grinker, R.R. (2011). Prevalence of autism spectrum disorder in a total population sample. American Journal of Psychiatry, 168, 904–912. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P. , & Risi, S. (2002). The Autism Diagnostic Observation Scale (ADOS). Los Angeles: Western Psychological Services. [Google Scholar]
- Maher, G.M. , O'Keeffe, G.W. , Kearney, P.M. , Kenny, L.C. , Dinan, T.G. , Mattsson, M. , & Khashan, A.S. (2018). Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: A systematic review and meta‐analysis. JAMA Psychiatry, 75, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell, D. , & Lecavalier, L. (2014). Should we believe the Centers for Disease Control and Prevention's autism spectrum disorder prevalence estimates? Autism, 18, 482–484. [DOI] [PubMed] [Google Scholar]
- Meilleur, A.A. , & Fombonne, E. (2009). Regression of language and non‐language skills in pervasive developmental disorders. Journal of Intellectual Disability Research, 53, 115–124. [DOI] [PubMed] [Google Scholar]
- Meyers, J. , Presmanes Hill, A. , Zuckerman, K. , & Fombonne, E. (2018). Autism spectrum disorders In Hollander E., Hagerman R., & Fein D. (Eds), Epidemiology (pp. 1–48). Washington, DC: American Psychiatric Association Publishing. [Google Scholar]
- Ministry of Development Planning & Statistics . (2015). First section: Population and social statistics. Available from: https://www.mdps.gov.qa/ [last accessed 1 September 2018].
- Ministry of Development Planning & Statistics . (2018a). Monthly figures on total population. Available from: https://www.mdps.gov.qa/ [last accessed 1 September 2018].
- Ministry of Development Planning & Statistics . (2018b). Qatar monthly statistics: statistics of January 2018: 49th issue. Available from: https://www.mdps.gov.qa/ [last accessed 1 September 2018].
- Ministry of Development Planning & Statistics . (2018c). Qatar monthly statistics – statistics of July 2018: 55th issue. Available from: https://www.mdps.gov.qa/ [last accessed 1 September 2018].
- Ministry of Education . (2016). Education statistical bulletin May 2016–2017. Available from: http://www.edu.gov.qa [last accessed 1 September 2018].
- Pantelis, P.C. , & Kennedy, D.P. (2015). Estimation of the prevalence of autism spectrum disorder in South Korea, revisited. Autism, 20, 517–527. [DOI] [PubMed] [Google Scholar]
- Pelly, L. , Vardy, C. , Fernandez, B. , Newhook, L.A. , & Chafe, R. (2015). Incidence and cohort prevalence for autism spectrum disorders in the Avalon Peninsula, Newfoundland and Labrador. Canadian Medical Association Journal (CMAJ) Open, 3, E276–E280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger, T.V. (1998). What's wrong with Bonferroni adjustments. BMJ, 316, 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles, A. , Simonoff, E. , Conti‐Ramsden, G. , Falcaro, M. , Simkin, Z. , Charman, T. , … & Baird, G. (2009). Loss of language in early development of autism and specific language impairment. Journal of Child Psychology and Psychiatry, 50, 843–852. [DOI] [PubMed] [Google Scholar]
- Randall, M. , Sciberras, E. , Brignell, A. , Ihsen, E. , Efron, D. , Dissanayake, C. , & Williams, K. (2016). Autism spectrum disorder: Presentation and prevalence in a nationally representative Australian sample. Australian and New Zealand Journal of Psychiatry, 50, 243–253. [DOI] [PubMed] [Google Scholar]
- Richler, J. , Luyster, R. , Risi, S. , Hsu, W.L. , Dawson, G. , Bernier, R. , … & Lord, C. (2006). Is there a ‘regressive phenotype’ of Autism Spectrum Disorder associated with the measles‐mumps‐rubella vaccine? A CPEA Study. Journal of Autism and Developmental Disorders, 36, 299–316. [DOI] [PubMed] [Google Scholar]
- Rothman, K.J. (2014). Six persistent research misconceptions. Journal of General Internal Medicine, 29, 1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter, M. , Bailey, A. , & Lord, C. (2003). The Social Communication Questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Rutter, M. , LeCouteur, A. , & Lord, C. (2003). The autism diagnostic interview‐revised (ADI‐R). Los Angeles: Western Psychological Services. [Google Scholar]
- Shattuck, P.T. , Durkin, M. , Maenner, M. , Newschaffer, C. , Mandell, D.S. , Wiggins, L. , & Cuniff, C. (2009). Timing of identification among children with an autism spectrum disorder: Findings from a population‐based surveillance study. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff, E. , Pickles, A. , Charman, T. , Chanldler, S. , Loucas, T. , & Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population‐derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2017). Stata statistical software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- Tadmouri, G.O. , Nair, P. , Obeid, T. , Al Ali, M.T. , Al Khaja, N. , & Hamamy, H.A. (2009). Consanguinity and reproductive health among Arabs. Reproductive Health, 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm, A. , Powell, E.M. , Neul, J.L. , Wagner, A. , & Zwaigenbaum, L. (2018). Loss of skills and onset patterns in neurodevelopmental disorders: Understanding the neurobiology mechanisms. Autism Research, 11, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . (2016). Education and literacy. Available from: https://data.unicef.org/topic/education/overview/ [last accessed 1 September 2018].
- Van Naarden Braun, K. , Pettygrove, S. , Daniels, J. , Miller, L. , Nicholas, J. , Baio, J. , … & Centers for Disease Control and Prevention (2007). Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. Morbidity and Mortality Weekly Report (MMWR) Surveillance Summary, 56, 29–40. [PubMed] [Google Scholar]
- Willsey, A.J. , & State, M.W. (2015). Autism spectrum disorders: From genes to neurobiology. Current Opinion on Neurobiology, 30, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury‐Smith, M. , & Scherer, S. (2018). Progress in the genetics of autism spectrum disorder. Journal of Developmental Medicine and Child Neurology, 60, 445–451. [DOI] [PubMed] [Google Scholar]
- Xu, G. , Jing, J. , Bowers, K. , Liu, B. , & Bao, W. (2014). Maternal diabetes and the risk of autism spectrum disorders in the offspring: A systematic review and meta‐analysis. Journal of Autism and Developmental Disorders, 44, 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeargin‐Allsopp, M. , Rice, C. , Karapurkar, T. , Doernberg, N. , Boyle, C. , & Murphy, C. (2003). Prevalence of autism in a US metropolitan area. Journal of the American Medical Association, 289, 49–55. [DOI] [PubMed] [Google Scholar]
- Zachor, D.A. , & Ben‐Itzchak, E. (2016). Specific medical conditions are associated with unique behavioral profiles in autism spectrum disorders. Frontiers of Neuroscience, 10, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Xu, X. , Yan, W. , Zou, X. , Wu, L. , Luo, X. , … & Jiang, Y. (unpublished data). Prevalence of autism spectrum disorder in China: A nationwide multi‐centre population‐based study among children aged 6 to 12 years. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Qatar Clinical Centers (QCC) sites.
Appendix S2. Other study features.
Appendix S3. Test‐retest reliability.
Table S1. Prior surveys of ASD in Arabic countries.
Table S2. Predictors of severity: results of univariate logistic regression models (N = 844).
Table S3. Predictors of ASD severity: final multivariate logistic model (N = 737).


