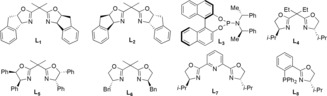
Table 2.
Optimization of the reaction conditions for the synthesis of enantioenriched chloro‐substituted allenylsilane (S)‐3 c.[a,b]

|
Entry |
Cat. (mol %) |
Ligand* (mol %) |
Base (2.0 equiv) |
Solvent |
T [°C] |
Yield [%] |
ee [c] [%] |
|---|---|---|---|---|---|---|---|
|
1 |
CuI (10) |
L1 (12) |
Et3N |
MeOH |
−10 |
29 |
47 |
|
2 |
CuI (10) |
L2 (12) |
Et3N |
MeOH |
−10 |
7 |
50 |
|
3 |
CuI (10) |
L3 (12) |
Et3N |
MeOH |
−10 |
91 |
0 |
|
4 |
CuI (10) |
L4 (12) |
Et3N |
MeOH |
−10 |
17 |
10 |
|
5 |
CuI (10) |
L6 (12) |
Et3N |
MeOH |
−10 |
32 |
10 |
|
6 |
CuI (10) |
L7 (12) |
Et3N |
MeOH |
−10 |
68 |
8 |
|
7 |
CuI (10) |
L8 (12) |
Et3N |
MeOH |
−10 |
27 |
2 |
|
8 |
CuI (10) |
L2 (12) |
Et3N |
EtOH |
−10 |
12 |
40 |
|
9 |
CuI (10) |
L2 (12) |
PPD |
MeOH |
−10 |
22 |
52 |
|
10 |
CuI (10) |
L5 (12) |
PPD |
MeOH |
−10 |
61 |
60 |
|
11 |
CuI (10) |
L5 (12) |
TMP |
MeOH |
−10 |
61 |
66 |
|
12 |
CuI (10) |
L5 (12) |
TMP |
MeOH |
−30 |
53 |
74 |
|
13 |
CuF2 (10) |
L5 (12) |
TMP |
MeOH |
−30 |
60 |
86 |
|
14 |
CuF2 (10) |
L5 (20) |
TMP |
MeOH |
−30 |
62 |
90 |
|
15 |
CuF2 (5) |
L5 (10) |
TMP |
MeOH |
−30 |
62 |
84 |
|
| |||||||
[a] Reaction was run under the following reaction conditions: 1 c (0.2 mmol), 2 (0.4 mmol, 2.0 equiv), base (0.4 mmol, 2.0 equiv), 10 mol % copper catalyst, and ligand in 1.0 mL of either anhydrous MeOH or EtOH at indicated temperature under argon atmosphere for corresponding time. [b] Yield of isolated product. [c] The ee values were determined by HPLC analysis. PPD=piperidine, TMP=2,2,6,6‐tetramethylpiperidiene.