Figure 1.
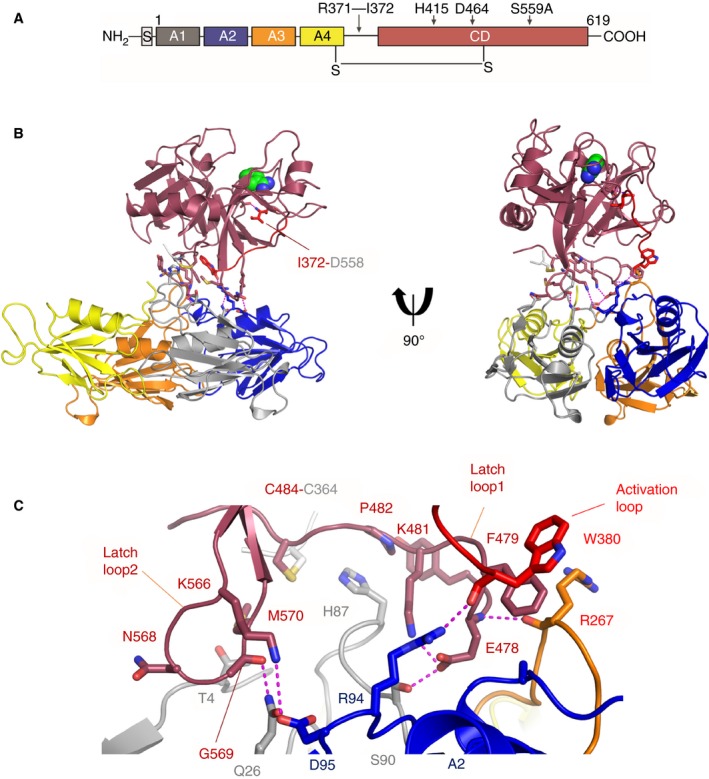
Structure of PKa. (A) Domain organization of plasma prekallikrein. The Arg371‐Ile372 cleavage site in the activation loop is indicated. (B) A cartoon diagram of the PKa structure shows two views related by a 90°‐rotation with the protease domain in dark red and the four apple domains colored as A1 (gray), A2 (blue), A3 (orange), and A4 (yellow). Benzamidine is shown as spheres (green) bound in the S1 pocket. The activation loop is red with Ile372 shown as sticks salt bridging to Asp558. Interfacial residues are shown as sticks with electrostatic and hydrogen bonds as dotted purple lines. (C) Close‐up view of the interface between the PKa protease domain and apple domain disc.
