Figure 1.
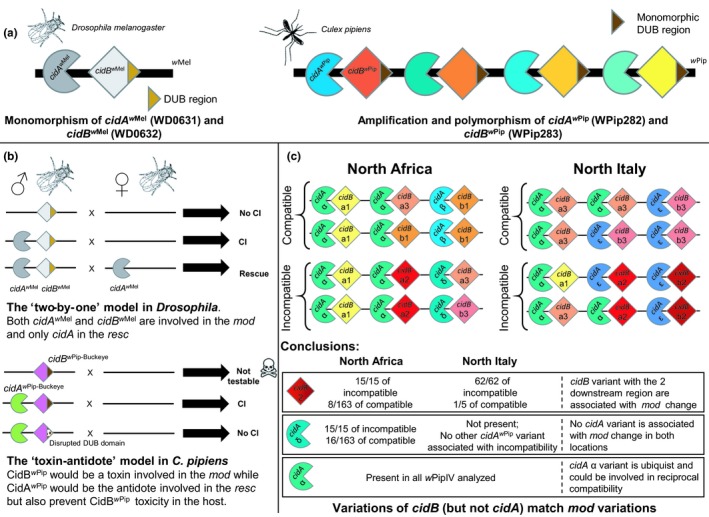
Summary of transgenic experiments and natural population studies conducted on cidA and cidB. (a) Hypothetical representation of the portion of prophage WO containing cidA and cidB CI genes in wMel and wPip Wolbachia strains. The genome of wMel contain only one copy of cidA and cidB genes (Lindsey et al., 2018). Genomes of all the wPip strains from several wPip groups investigated contain several different copies of cidA and cidB genes (Bonneau, Atyame, et al., 2018). The deubiquitinase (DUB) region is the catalytic domain of CidB protein (Beckmann et al., 2017). This region is conserved between the cidBw Pip variants (Bonneau, Atyame, et al., 2018). (b) transgenic expression of cidA and cidB from either wMel or wPip‐Buckeye genomes in Drosophila melanogaster flies. The expression of both cidAw Mel and cidBw Mel in D. melanogaster males is required to induce CI, neither cidAw Mel or cidBw Mel alone can induce CI (LePage et al., 2017; Shropshire & Bordenstein, 2019). The expression of cidAw Mel alone in D. melanogaster females is sufficient to rescue CI (Shropshire et al., 2018). A two‐by‐one model of CI was proposed in D. melanogaster in which cidAw Mel acts as a mod factor when expressed in males and as a resc factor when expressed in females (Shropshire & Bordenstein, 2019; Shropshire et al., 2018). The production of viable transgenic male flies expressing only cidBw Pip was not possible suggesting a toxic effect of the CidBw Pip protein (represented by a skull) while flies expressing both cidAw Pip and cidBw Pip were viable and capable of CI induction. The male flies expressing cidAw Pip and cidBw Pip with a disrupted catalytic DUB domain were not capable to induce CI suggesting that the DUB region is functionally involved in CI. The current transgenic data in Culex pipiens support a toxin‐antidote model where cidBw Pip would encode a toxin involved in the mod function while cidAw Pip would encode the antidote involved in the resc but also prevent the producer from the toxicity of CidBw Pip protein. (c) cidAw Pip and cidBw Pip variants repertoires in natural populations of C. pipiens infected with wPipIV strains. Full variant names are not shown (they all belong to the group wPipIV) and only the letter/number of the variant appear. For instance cidA α referring to cidA_IV(α) and cidB a2 to cidB_IV(a/2). The number of pairs of genes as well as their disposition in the genome might not reflect the reality as these informations are still under investigation. Males from North African and North Italian natural populations are either compatible or incompatible with females from the Tunis isofemale line depending on the wPipIV strain they carry. All the wPipIV strains carry several cidAwPip and cidBwPip variants inside their genomes. The variants cidB_IV(2) [i.e., cidB_IV(a/2) and cidB_IV(b/2)] were found associated with the incompatible mod phenotype in both geographical areas while the variant cidA_IV(δ) was found associated with the incompatible mod phenotype only in the North African population. Furthermore, no other cidA variant or combination of variants was found associated with mod phenotype variation in North Italian populations suggesting that only cidB plays a role in the mod phenotype variations in C. pipiens. Finally, the cidA_IV(α) variant was detected in all the wPipIV strains regardless of their mod phenotype and their geographical origins. The ubiquity of the cidA_IV(α) variant could be responsible for the reciprocal compatibility always observed between mosquitoes infected with different wPipIV strains and suggests a role of cidA in the resc function [Colour figure can be viewed at http://www.wileyonlinelibrary.com]
