Figure 1.
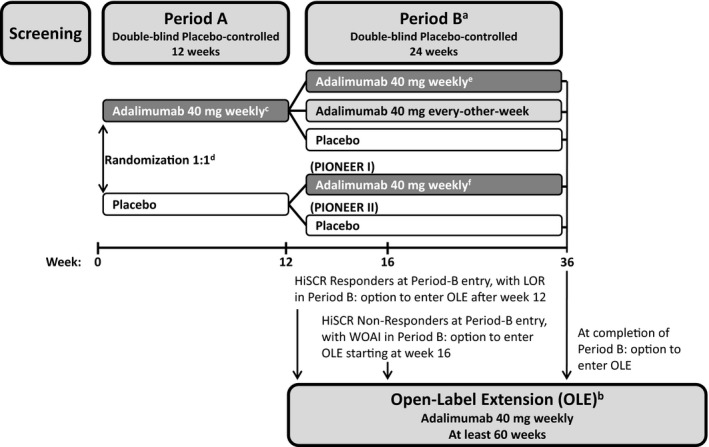
Study design.
aWeek‐12 Hidradenitis Suppurativa (HS) Clinical Response (HiSCR) responders through Period B to week 36 or until loss of response [loss of 50% of the abscess and inflammatory nodule (AN) count improvement gained between baseline and week 12], and week‐12 HiSCR nonresponders continued Period B to at least week 26 (and up to week 36). bPatients could enter the multicentre 60‐week phase III OLE trial (which evaluated long‐term safety, tolerability and efficacy of adalimumab for patients with moderate‐to‐severe HS), if (i) they completed Period B of their respective PIONEER trial; (ii) achieved HiSCR at entry to Period B of their respective PIONEER trial and then experienced a loss of response (LOR); or (iii) did not achieve HiSCR at the entry of Period B and then experienced worsening or absence of improvement (WOAI) (greater or equal to the baseline AN count on two consecutive visits after week 12, occurring at least 14 days apart). cStarting at week 4 after 160 mg (week 0), 80 mg (week 2). dStratified by baseline Hurley stage II vs. Hurley stage III (PIONEER I and II) and baseline concomitant antibiotic use (PIONEER II). eRerandomization for patients treated with adalimumab in Period A was stratified by week‐12 HiSCR status at entry into Period B and by baseline Hurley stage II vs. Hurley stage III. fAdalimumab 40 mg starting at week 16 after 160 mg (week 12), 80 mg (week 14).
