Figure 3.
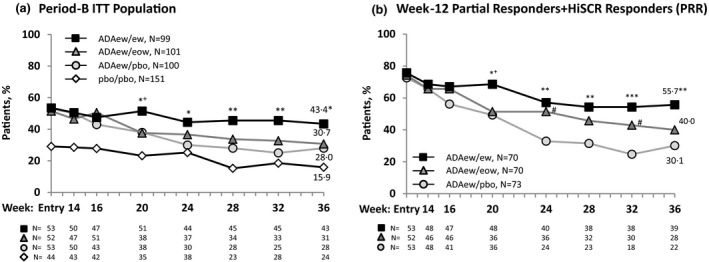
Achievement of Hidradenitis Suppurativa Clinical Response (HiSCR) at week 36 for patients who received adalimumab (ADA) every week (ew) in Period A. (a) Period‐B intention‐to‐treat population. Overall, patients rerandomized to ADAew did better in Period B than those rerandomized to ADA every other week (eow) or placebo (pbo). The pbo/pbo group was included only in the PIONEER II study; hence, a comparison between the other treatment groups and the pbo/pbo group was not performed (it is shown here for illustration purposes only). (b) Week‐12 partial responder plus HiSCR responders (PRR). This population was identified as the patients most likely to benefit from weekly adalimumab treatment continued past week 12. Nonresponder imputation. Statistical significance at ***P = 0·001, **P = 0·01, *P = 0·05 for ADAew/ew vs. ADA ew/pbo; + P = 0·05 for ADAew/ew vs. ADAew/eow; and # P = 0·05 for ADAew/eow vs. ADAew/pbo. P‐values were calculated using the Cochran–Mantel–Haenszel test adjusted for study, baseline Hurley stage and HiSCR responder at entry of Period B (Fig. 3a), and HiSCR status at rerandomization (Fig. 3b).
