Figure 4.
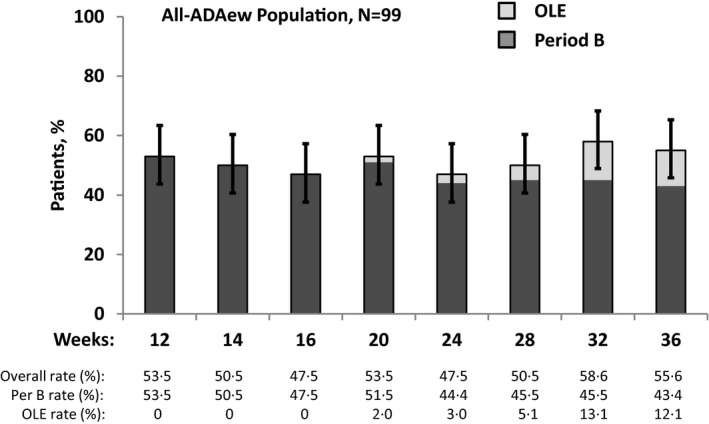
Maintenance of Hidradenitis Suppurativa Clinical Response (HiSCR) for patients receiving continuous adalimumab (ADA) every week (ADAew) across PIONEER I, II and open‐label extension (OLE). Patients in this analysis are those who received ADAew in both study periods (all‐ADAew population, N = 99). This analysis includes data from both study periods, in addition to the OLE data for the all‐ADAew population who discontinued Period B owing to loss of response, entered the OLE, and regained response in the OLE. This demonstrates maintenance of treatment response for patients who may regain response following temporary loss of response.
