Figure 5.
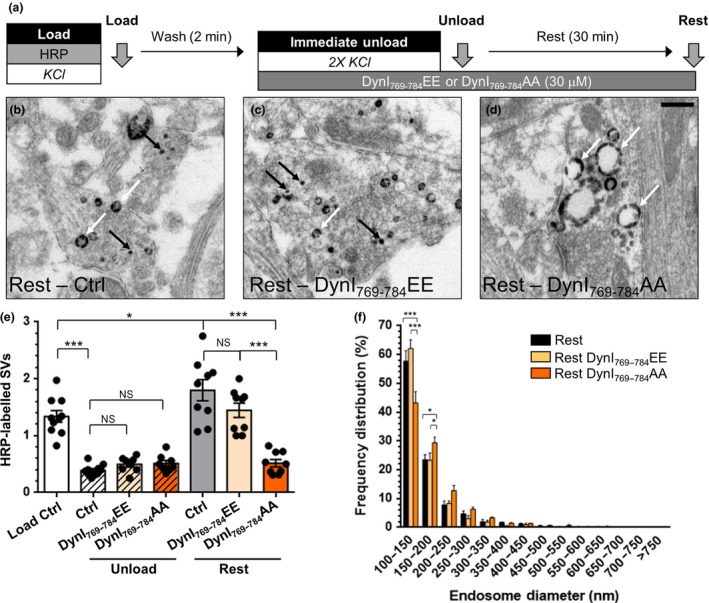
Disruption of the dynamin I–syndapin I interaction arrests synaptic vesicle (SV) budding from bulk endosomes. (a) Cultures were loaded with horse radish peroxidase (HRP) (10 mg/mL) for 2 min in the presence of KCl (50 mM) and washed immediately to remove excess HRP. Where indicated cultures were incubated with either DynI769–784AA or DynI769–784EE peptides (both 30 µM) immediately after this wash step. Neurons were then stimulated twice with KCl (50 mM, 30 s each) to release all available SVs (Immediate Unload) and left to rest for 30 min. Cultures were either fixed after HRP loading (Load), the immediate unload (Unload) or the rest period (Rest) as indicated by arrows. (b–d) Representative electron micrographs of the treatments described above are shown (b – Rest, c – Rest DynI769–784EE, d – Rest DynI769–784AA; scale bar – 500 nm). Black and white arrows indicate HRP‐labelled SVs and endosomes respectively. (e) Bar graph displays the mean number of HRP‐labelled SVs per nerve terminal ± SEM in either Load, Immediate Unload (hatched bars) and Rest (solid bars) conditions in the presence or absence of peptides. Number of experiments: n = 10 for Load Ctrl and Unload Ctrl, n = 9 for Rest Ctrl, Rest DynI769–784EE and Rest DynI769–784AA, n = 8 for Unload DynI769–784EE and Rest DynI769–784AA from three culture preparations *p < 0.05, ***p < 0.001 one‐way anova). (f) Frequency distribution of endosome diameter for Rest with competitive peptides. The number of HRP‐labelled endosomes were as follows: Rest Ctrl n = 1585; Rest DynI769–784EE n = 801; Rest DynI769–784AA n = 1571, n = 9 coverslips for Rest Ctrl, Rest DynI769–784EE and Rest DynI769–784AA from three culture preparations (*p < 0.05, ***p < 0.001, two‐way anova).
