Figure 1.
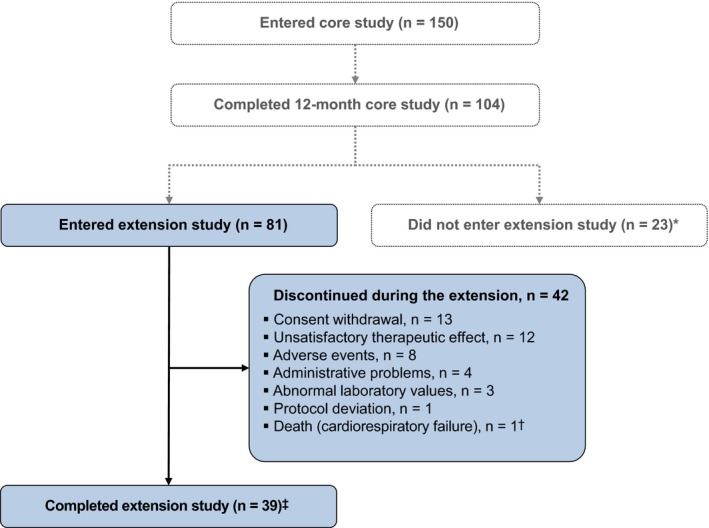
Patient disposition. *Reasons for not entering the extension were not recorded; †The death occurred 16 d after the 16th injection and was not suspected by the treating physician to be related to pasireotide11; ‡Patients were considered to have completed the extension if both of the following criteria were met: they had received an additional ≥12 mo of treatment during the extension; and the core study had been unblinded, which occurred once all 150 patients who were enrolled in the core study had reached month 12 or discontinued treatment. The AEs leading to discontinuation in 8 patients during the extension phase were as follows: diabetes mellitus, n = 2; hyperglycaemia, n = 3 (one of these patients also had increased gamma‐glutamyltransferase); hyperkalaemia, n = 1; endometrial cancer, n = 1; and acute cholecystitis, cholelithiasis, elevated liver enzymes, bilirubin increased, oedematous pancreatitis and ascites, n = 1 [Colour figure can be viewed at http://wileyonlinelibrary.com]
