Abstract
Objective
To assess the frequency of venous thromboembolism (VTE) events in the Rituximab in Antineutrophil Cytoplasmic Antibody (ANCA)–Associated Vasculitis (RAVE) trial and identify novel potential risk factors.
Methods
VTE events in 197 patients enrolled in the RAVE trial were analyzed. Baseline demographic and clinical characteristics were recorded, and univariate and multivariate analyses were performed to identify factors associated with VTE in ANCA‐associated vasculitis (AAV).
Results
VTE occurred in 16 patients (8.1%) with an overall average time to event of 1.5 months (range 1.0–2.75). In univariate analyses with calculation of hazard ratios (HRs) and 95% confidence intervals (95% CIs), heart involvement (HR 17.408 [95% CI 2.247–134.842]; P = 0.006), positive proteinase 3 (PR3)–ANCA (HR 7.731 [95% CI 1.021–58.545]; P = 0.048), pulmonary hemorrhage (HR 3.889 [95% CI 1.448–10.448]; P = 0.008), and the presence of red blood cell casts (HR 15.617 [95% CI 3.491–69.854]; P < 0.001) were associated with the onset of VTE. In multivariate models adjusted for age and sex, the significant associations between VTE events and heart involvement (HR 21.836 [95% CI 2.566–185.805]; P = 0.005), PR3‐ANCA (HR 9.12 [95% CI 1.158–71.839]; P = 0.036), pulmonary hemorrhage (HR 3.91 [95% CI 1.453–10.522]; P = 0.007), and urinary red blood cell casts (HR 16.455 [95% CI 3.607–75.075]; P < 0.001) remained.
Conclusion
Patients diagnosed as having AAV with pulmonary hemorrhage, positive PR3‐ANCA, heart involvement, and the presence of red blood cell casts are at an increased risk to develop VTE. Further studies are needed to confirm and expand these findings and to explore the mechanisms of hypercoagulability in these patients with the aim of informing potential targets for therapeutic intervention.
Introduction
The therapeutic methods available to treat antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAVs) expanded with the approval of rituximab (RTX) as an alternative therapy to cyclophosphamide (CYC) as the induction treatment for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) 1, 2. As treatment approaches and patient survival have improved over the past decades, longer‐term outcome and complications attributable to either the disease or immunosuppressive therapy have moved into the research focus. Consequently, recent reports have highlighted an increased frequency of venous thromboembolism (VTE) events in patients with AAV. Analysis of a randomized controlled trial that included patients with GPA enrolled in the Wegener's Granulomatosis Etanercept Trial (WGET) demonstrated an incidence of VTE of 7.0 per 100 person‐years 3. An increased likelihood of VTE was reported in a population‐based incident AAV cohort, which was driven by a significantly increased risk of developing deep venous thrombosis (DVT) 4. Analysis of a large cohort of patients with eosinophilic granulomatosis with polyangiitis (EGPA), GPA, and MPA demonstrated occurrence of VTE in 8.2%, 8.0%, and 7.8% of patients, respectively 5. More recently, analysis of data derived from several trials conducted by the European Vasculitis Society showed the occurrence of VTE in 41 (9.8%) of 417 patients with GPA or MPA 6. While VTE is now acknowledged as a commonly occurring complication of AAV, its pathogenesis remains ill‐defined. Several factors have been considered to play a role in VTE pathogenesis, including the presence of antiplasminogen antibodies 7 and excess thrombin generation facilitated by tissue factor, microparticles, and neutrophil extracellular traps 8.
The aim of the current study was to further explore the relationship between VTE and AAV through analysis of data from the Rituximab in ANCA‐Associated Vasculitis (RAVE) trial 1. This trial allowed for prospective follow‐up of patients with GPA and MPA and presented the first opportunity to study the impact of 2 different induction treatment strategies, namely RTX and CYC, on the occurrence of VTE events.
Patients and Methods
Study design and treatment regimens
The RAVE study was a double‐blind, placebo‐controlled trial in which 197 patients were randomized to receive either RTX (375 mg/m2 weekly for 4 weeks; n = 99) or CYC (2 mg/kg body weight for 3–6 months) followed by maintenance treatment with azathioprine (2 mg/kg body weight, maximum dosage 150 mg/day; n = 98). Glucocorticoids were tapered and withdrawn within 5.5 months in both groups. Detailed trial design and the respective results for short‐ and the long‐term follow‐up have been previously described 1, 9.
Definitions of outcome variables
Patients were classified according to their AAV diagnosis (GPA or MPA) based on the 1994 Chapel Hill Consensus Conference Nomenclature for Vasculitis 10. Patients were further classified according to either proteinase 3 (PR3)–ANCA or myeloperoxidase (MPO)–ANCA. Information related to patient demographic characteristics, newly diagnosed/relapsing disease, specific organ involvement, treatment, and outcome was collected. Vasculitis activity was assessed using the Birmingham Vasculitis Activity Score for Wegener's Granulomatosis (BVAS/WG) 11, and the Vasculitis Damage Index was used to grade the respective damage 12. The occurrence of either DVT, pulmonary embolism (PE), or both was considered a VTE event.
Statistical analysis
For baseline characteristics, the number and percent of patients, mean ± SD, or median with interquartile range was used for analysis, as appropriate. Characteristics between patients with and those without VTE were compared by Student's t‐test or Mann‐Whitney test for continuous data and chi‐square test or Fisher's exact test for categorical data. Spearman's correlation coefficient was used to report the relationship between VTE and named factors or states.
Kaplan‐Meier curves were plotted for the occurrence of first VTE events and significant variables. Survival curves were compared by log rank test. Univariate Cox regression models were used to assess the effect of factors and states in patients with and those without VTE. For every significant parameter in univariate analyses, a multivariate Cox regression model with adjustment for age and sex was applied. Findings of the univariate and multivariate analyses were assessed using hazard ratios (HRs) and 95% confidence intervals (95% CIs). P values less than 0.05 were considered significant.
Results
Patient characteristics stratified by VTE
Of the 197 patients enrolled in the RAVE trial, 42 (21.3%) received anticoagulation therapy as either thromboprophylaxis or due to an earlier medical history necessitating anticoagulation treatment (such as atrial fibrillation). VTE events were recorded in 16 patients. Of these, 11 patients had DVT, 4 had PE, and 1 had concomitant DVT and PE. Overall, the average time from baseline to VTE was 1.5 months (range 1.0–2.75) (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41017/abstract).
A higher BVAS/WG at baseline was recorded for patients with VTE versus those without VTE (HR 10 [95% CI 9–11.75] versus 8 [95% CI 6–10]; P = 0.017). We observed a higher frequency of VTE events among patients with PR3‐ANCA (15 events among 133 patients) than among those with MPO‐ANCA (1 event among 64 patients) (P = 0.016). Moreover, pulmonary hemorrhage and heart involvement were more frequent in the VTE group (P = 0.004 and P = 0.001, respectively). Of the 16 VTE events, 7 occurred in RTX‐treated patients and 9 in CYC‐treated patients (P = 0.558). In contrast, sinusitis was less frequently observed in patients who experienced a VTE event than those who did not (31.3% versus 56.9%; P = 0.048) (see Supplementary Table 2, http://onlinelibrary.wiley.com/doi/10.1002/art.41017/abstract). A positive correlation was observed between VTE and heart involvement (r = 0.24, P < 0.01), pulmonary hemorrhage (r = 0.21, P < 0.01), positive PR3‐ANCA (r = 0.17, P < 0.05), BVAS/WG (r = 0.17, P < 0.05), and the presence of red blood cell (RBC) casts (r = 0.373, P < 0.001). There was a negative association between VTE and eye involvement (r = –0.16, P < 0.05) and sinusitis (r = –0.14, P < 0.05) (see Supplementary Table 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.41017/abstract). Kaplan‐Meier curves for pulmonary hemorrhage, positive PR3‐ANCA, and RBCs are shown in Figure 1.
Figure 1.
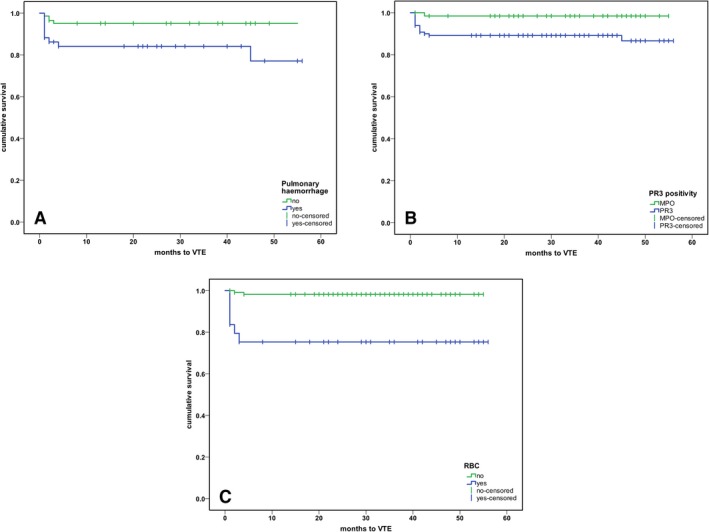
Kaplan‐Meier analysis of the association between venous thromboembolism (VTE) and pulmonary hemorrhage, proteinase 3 (PR3) positivity, and the presence of red blood cell (RBC) casts. A, Event‐free survival of patients with and patients without pulmonary hemorrhage (P < 0.003). B, Time to VTE event according to the specific antigen (PR3 versus myeloperoxidase [MPO]) (P < 0.018). C, Time to VTE event in patients with RBC casts compared to those without significant RBC casts (P < 0.001). All analyses were calculated from trial entry.
Predictors of VTE
Univariate analyses were performed to identify variables associated with VTE. Among clinical factors found to have significant values, we demonstrated heart involvement (HR 17.408 [95% CI 2.247–134.842]; P = 0.006), PR3 positivity (HR 7.731 [95% CI 1.021–58.545]; P = 0.048), pulmonary hemorrhage (HR 3.889 [95% CI 1.448–10.448]; P = 0.008), and presence of RBCs (HR 15.617 [95% 3.491–69.854]; P < 0.001) to be positively associated with VTE events. The frequency of VTE was similar in both treatment groups (RTX versus CYC) (HR 0.747 [95% 0.278–2.006]; P = 0.563), and there was no difference between newly diagnosed patients and patients with relapsing disease (HR 1.067 [95% CI 0.400–2.844]; P = 0.896) (Table 1). Multivariate models adjusted for age and sex were used to confirm the associations identified by univariate analyses. After adjustment for demographic variables, the associations between VTE and the presence of heart involvement (HR 21.836 [95% CI 2.566–185.805]; P = 0.005), pulmonary hemorrhage (HR 3.91 [95% CI 1.453–10.522]; P = 0.007), PR3 positivity (HR 9.12 [95% CI 1.158–71.839]; P = 0.036), and presence of RBCs (HR 16.455 [95% CI 3.607–75.075]; P < 0.001) remained significant (Table 2).
Table 1.
Univariate Cox regression results indicating the association between clinical variables and VTE events in 197 patients with antineutrophil cytoplasmic antibody–associated vasculitis*
| Variable | Median value or % of patients | HR (95% CI) | P |
|---|---|---|---|
| Demographics and baseline clinical characteristics | |||
| Age, median years | 52 | 1.005 (0.973–1.039) | 0.745 |
| Body mass index, median kg/m2 | 27.6 | 1.042 (0.974–1.116) | 0.229 |
| Creatinine, median mg/dl | 1.2 | 1.209 (0.524–2.167) | 0.524 |
| BVAS/WG score, median | 8 | 1.121 (0.994–1.264) | 0.063 |
| C‐reactive protein, median mg/dl | 3.03 | 1.051 (0.960–1.150) | 0.282 |
| GPA vs. MPA, % | 74.7 | 1.941 (0.529–7.125) | 0.317 |
| Newly diagnosed disease vs. relapsing disease, % | 48.7 | 1.067 (0.400–2.844) | 0.896 |
| PR3 vs. MPO positivity, % | 66.5 | 7.731 (1.021–58.545) | 0.048 |
| Proteinuria (dipstick), % | 72.1 | 0.675 (0.358–1.274) | 0.226 |
| Serum albumin, median gm/dl | 3.75 | 1.353 (0.549–3.333) | 0.511 |
| Male sex, % | 50.7 | 1.319 (0.491–3.542) | 0.583 |
| Disease manifestations, % | |||
| Arthralgia | 56.3 | 0.988 (0.368–2.653) | 0.981 |
| Eye involvement | 23.4 | 0.032 (0.000–4.322) | 0.169 |
| Heart involvement | 0.5 | 17.408 (2.247–134.842) | 0.006 |
| Peripheral nervous system involvement | 19.3 | 0.966 (0.275–3.391) | 0.957 |
| Pulmonary hemorrhage | 25.9 | 3.889 (1.448–10.448) | 0.008 |
| Red blood cell casts | 23.8† | 15.617 (3.491–69.854) | <0.001 |
| Renal involvement‡ | 28.5 | 2.238 (0.638–7.855) | 0.208 |
| Sinusitis | 54.8 | 0.355 (0.123–1.023) | 0.055 |
| Skin involvement | 17.3 | 1.634 (0.527–5.070) | 0.395 |
| VDI at baseline, median | 1.18 | 0.793 (0.528–1.192) | 0.265 |
| Treatment modalities, % | |||
| Antihypertensive agents | 100 | 1.189 (0.788–1.794) | 0.410 |
| Diuretic use (single agent) | 24.9 | 1.389 (0.482–4.000) | 0.542 |
| Methylprednisolone bolus | 64.5 | 0.415 (0.154–1.114) | 0.081 |
| Rituximab vs. cyclophosphamide | 50.8 | 0.747 (0.278–2.006) | 0.563 |
| Sequential nephron blockade (loop and thiazide diuretics) | 2.5 | 1.001 (0.623–1.609) | 0.995 |
| Cumulative steroid dose (6 months), median gm | 3.99 | 0.673 (0.426–1.064) | 0.090 |
| Follow‐up variables, % | |||
| Infection | 82.2 | 1.526 (0.347–6.716) | 0.526 |
| Malignancy | 3.6 | 0.047 (0.000–4,691.49) | 0.603 |
| Relapse | 40.1 | 1.437 (0.539–3.831) | 0.511 |
| VDI change (>1)§ | 0.998 (0.817–1.219) | 0.984 |
VTE = venous thromboembolism; HR = hazard ratio; 95% CI = 95% confidence interval; BVAS/WG = Birmingham Vasculitis Activity Score for Wegener's Granulomatosis; GPA = granulomatosis with polyangiitis; MPA = microscopic polyangiitis; PR3 = proteinase 3; MPO = myeloperoxidase; VDI = Vasculitis Damage Index.
Data were available for 165 patients.
Renal involvement was defined as the presence of significant hematuria, red blood cell casts, or reduced creatinine clearance (attributable to active vasculitis).
Excluding pulmonary infarction and complicated venous thrombosis.
Table 2.
Clinical associations with venous thromboembolism events in antineutrophil cytoplasmic antibody–associated vasculitis, determined by multivariate analysis of variables that had significant associations in the univariate analysis*
| Variable | Hazard ratio (95% CI) | P |
|---|---|---|
| Heart involvement | 21.836 (2.566–185.805) | 0.005 |
| Pulmonary hemorrhage | 3.910 (1.453–10.522) | 0.007 |
| Proteinase 3 positivity | 9.120 (1.158–71.839) | 0.036 |
| Red blood cell casts | 16.455 (3.607–75.075) | <0.001 |
Multivariate Cox regression models were adjusted for age and sex. 95% CI = 95% confidence interval.
Discussion
A hallmark of some systemic autoimmune diseases is their association with thrombosis 13. Consistent with this, several studies have shown that patients with AAV are at increased risk of developing thrombosis 3, 5, 13. When analyzing data from the RAVE trial, we observed VTE events in 8.1% of patients enrolled in the study. A majority of events (12 of 16) was recorded within the first 2 months of enrollment, highlighting the role of inflammation and presumably active disease in the onset of VTE. These findings align with previous results from the WGET trial, which indicated an increased risk of VTE in this patient population and a median time to event onset of 2.1 months 3. Moreover, we identified a trend toward an association between VTE and disease activity. A recent analysis of 417 patients from several randomized trials demonstrated an association between higher disease activity and VTE events 6, reinforcing the role of active vasculitis in predisposing individuals to thrombosis.
Several mechanisms may contribute to the procoagulable state in AAV. Antiplasminogen antibody titers have been shown to be higher in patients with PR3‐positive vasculitis compared to patients with either MPO‐positive vasculitis or idiopathic thrombosis, and these antibodies were detected in 5 of 9 vasculitis patients with thrombosis and PR3‐ANCA positivity compared to none of 4 patients with thrombosis and MPO‐ANCA positivity 7. Antiplasminogen antibodies also reacted with a highly restricted motif on plasminogen 7, indicating their potential to impair fibrinolysis, which may explain our findings demonstrating a higher frequency of VTE in patients positive for PR3‐ANCA. Moreover, patients with GPA exhibit an increased frequency of anticardiolipin antibodies when compared to the general population 14. The association between pulmonary hemorrhage and VTE in patients with AAV has not been previously described. A case series of patients with severe pulmonary hemorrhage due to systemic vasculitis showed a concomitant VTE event in 7 of 35 patients 15. It is tempting to speculate that these patients are prone to develop thrombosis due to more severe disease, as shown in our study. We found an association between the presence of urinary RBC casts and VTE, while renal involvement in general was not associated with VTE. One can speculate that presence of RBC casts is related to active glomerular inflammation and that inflammation in itself is triggering VTE.
Our work has several strengths and limitations. Since the RAVE study was a randomized controlled trial, the quality and completeness of the data (which are publicly accessible) are more appropriate compared to most other studies investigating VTE. Among the study's limitations, we only assessed clinically apparent VTE events (i.e., not investigated in a protocolized, prospective manner), and the definition of alveolar hemorrhage was left to site investigators. Due to the low number of events, adjustment for multiple comparisons was restricted to age and sex. Moreover, data on some variables, such as serum albumin and proteinuria (measured by dipstick), were not available for every participant.
In conclusion, our post hoc analysis of a prospective randomized controlled trial of patients with AAV has identified several factors associated with VTE risk. Patients with pulmonary hemorrhage may be prone to VTE. We identified PR3 positivity as a novel risk factor, which might be related to more frequent presence of antiplasminogen antibodies. The association between heart involvement and presence of RBCs has not been previously described and may reflect the contribution of overall disease burden to the risk of thrombotic complications. Further studies are needed to confirm and expand these findings and to explore the mechanisms of hypercoagulability in these patients with the aim of informing potential targets for therapeutic interventions.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Kronbichler had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Kronbichler, Leierer, Shin, Ytterberg, Stone.
Acquisition of data
Kronbichler, Leierer, Shin, Merkel, Spiera, Seo, Langford, Hoffman, Kallenberg, St. Clair, Brunetta, Fervenza, Geetha, Keogh, Monach, Ytterberg, Mayer, Specks, Stone.
Analysis and interpretation of data
Kronbichler, Leierer, Shin, Ytterberg, Stone.
Supporting information
Acknowledgments
We express our gratitude to all patients who participated in the study.
http://ClinicalTrials.gov identifier: NCT00104299 post‐results.
Supported by the Immune Tolerance Network (NIH contract N01‐AI‐15416; protocol ITN021AI), the National Institute of Allergy and Infectious Diseases, NIH, as well as the Juvenile Diabetes Research Foundation, Genentech Inc., and Biogen Idec. Work performed at Johns Hopkins University was supported by the NIH (grants K23‐AR‐052820 to Dr. Seo and K24‐AR‐049185 to Dr. Stone and National Center for Research Resources [NCRR] grant UL1‐RR‐025005). Work performed at Cleveland Clinic was supported by the NIH (National Center for Advancing Translational Sciences grant UL1‐TR‐00043). Work performed at the Mayo Clinic was supported by the Clinical and Translational Science Award (CTSA) Program (NCRR grant 1‐UL1‐RR‐024150‐01). Dr. Monach's work was supported by the CTSA Program (grants UL1‐RR‐025771, M01‐RR00533, and K24‐AR‐02224) and an Arthritis Foundation Investigator Award.
Drs. Kronbichler and Leierer contributed equally to this work.
Dr. Kronbichler has received speaking fees from Chugai, Terumo BCT, and Novartis (less than $10,000 each). Dr. Merkel has received consulting fees from ChemoCentryx and InflaRx (more than $10,000 each) and from AbbVie, AstraZeneca, Biogen, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Celgene, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, Insmed, Janssen, and Kiniksa (less than $10,000 each). Dr. Brunetta owns stock or stock options in Genentech. Dr. Monach has received consulting fees from Celgene and ChemoCentryx (less than $10,000 each). Dr. Mayer has received consulting fees and speaking fees from Boehringer‐Ingelheim, AbbVie, TEVA, Amgen, Novo Nordisk, Eli Lilly, Vifor, and Otsuka (less than $10,000 each). Dr. Stone has received consulting fees from Roche/Genentech (more than $10,000 each). No other disclosures relevant to this article were reported.
References
- 1. Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA‐associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA‐associated renal vasculitis. N Engl J Med 2010;363:211–20. [DOI] [PubMed] [Google Scholar]
- 3. Merkel PA, Lo GH, Holbrook JT, Tibbs AK, Allen NB, Davis JC Jr, et al, for the Wegener's Granulomatosis Etanercept Trial Research Group . High incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener's Clinical Occurrence of Thrombosis (WeCLOT) study. Ann Intern Med 2005;142:620–6. [DOI] [PubMed] [Google Scholar]
- 4. Berti A, Matteson EL, Crowson CS, Specks U, Cornec D. Risk of cardiovascular disease and venous thromboembolism among patients with incident ANCA‐associated vasculitis: a 20‐year population‐based cohort study. Mayo Clin Proc 2018;93:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allenbach Y, Seror R, Pagnoux C, Teixeira L, Guilpain P, Guillevin L, et al, for the French Vasculitis Study Group . High frequency of venous thromboembolic events in Churg‐Strauss syndrome, Wegener's granulomatosis and microscopic polyangiitis but not polyarteritis nodosa: a systematic retrospective study on 1130 patients. Ann Rheum Dis 2009;68:564–7. [DOI] [PubMed] [Google Scholar]
- 6. Kronbichler A, Leierer J, Leierer G, Mayer G, Casian A, Höglund P, et al. Clinical associations with venous thromboembolism in anti‐neutrophil cytoplasm antibody‐associated vasculitides. Rheumatology (Oxford) 2017;56:704–8. [DOI] [PubMed] [Google Scholar]
- 7. Bautz DJ, Preston GA, Lionaki S, Hewins P, Wolberg AS, Yang JJ, et al. Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3‐ANCA vasculitis. J Am Soc Nephrol 2008;19:2421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kambas K, Chrysanthopoulou A, Vassilopoulos D, Apostolidou E, Skendros P, Girod A, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis 2014;73:1854–63. [DOI] [PubMed] [Google Scholar]
- 9. Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission‐induction regimens for ANCA‐associated vasculitis. N Engl J Med 2013;369:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum 1994;37:187–92. [DOI] [PubMed] [Google Scholar]
- 11. Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al, for the International Network for the Study of the Systemic Vasculitides (INSSYS) . A disease‐specific activity index for Wegener's granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum 2001;44:912–20. [DOI] [PubMed] [Google Scholar]
- 12. Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997;40:371–80. [DOI] [PubMed] [Google Scholar]
- 13. Zöller B, Li X, Sundquist J, Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow‐up study from Sweden. Lancet 2012;379:244–9. [DOI] [PubMed] [Google Scholar]
- 14. Sebastian JK, Voetsch B, Stone JH, Romay‐Penabad Z, Lo GH, Allen NB, et al. The frequency of anticardiolipin antibodies and genetic mutations associated with hypercoagulability among patients with Wegener's granulomatosis with and without history of a thrombotic event. J Rheumatol 2007;34:2446–50. [PubMed] [Google Scholar]
- 15. De Sousa E, Smith R, Chaudhry A, Willcocks L, Jayne D. Venous thromboembolism with concurrent pulmonary haemorrhage in systemic vasculitis. Nephrol Dial Transplant 2012;27:4357–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


