Figure 2.
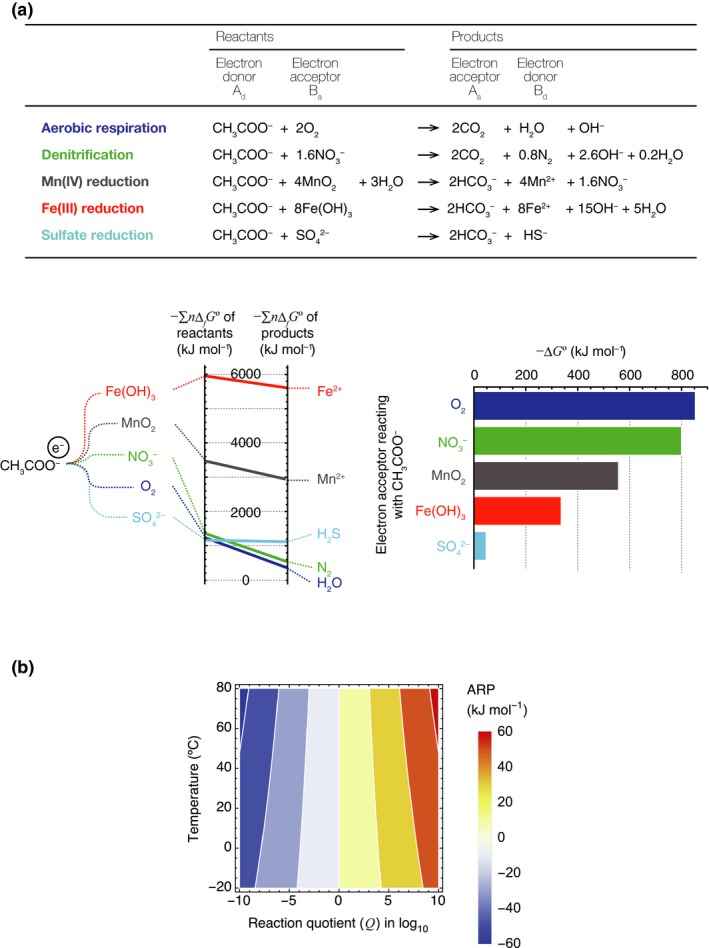
The first and second terms of the change in Gibbs energy in eqn 2a. (a) The negative change in the standard Gibbs energy, −∆G°, for a reaction that utilises CH3COO− as an electron donor with various electron acceptors. These examples of reactions and the values of −∆G° (at 273.15 K, 1 atm and pH = 7) were adapted from Konhauser (2007). −∆G° corresponds to the difference between the sum of the change in the standard Gibbs energy of formation of the reactants multiplied by their stoichiometric coefficient niΔfGº and the same for the by‐products (see Box. 1). −∆G° at constant temperature and pressure is determined by the combination of reactants and by‐products. (b) How the magnitude of the abundant resource premium (ARP = RT ln Q) varies with the reaction quotient Q and the ambient temperature T.
