Figure 1.
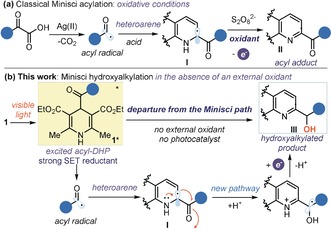
a) Classical Minisci reaction of acyl radicals with N‐heteroarenes where intermediate I is oxidized by an external oxidant to form the acylated product II. b) Diverting from the classical Minisci‐type chemistry: the photochemistry of 4‐acyl‐1,4‐dihydropyridines 1 secures the generation of acyl radicals in the absence of an external oxidant, thus triggering an unusual hydroxyalkylation of N‐heteroarenes; SET; single‐electron transfer.
