Figure 7.
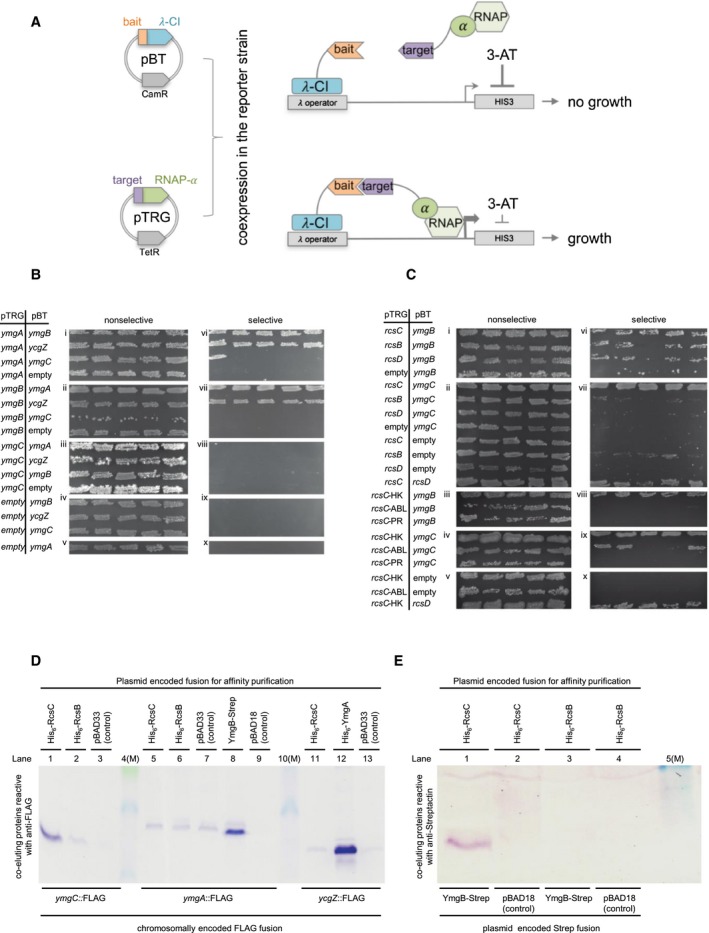
A complex formed by YmgA, YmgB and YcgZ interacts with RcsC.
A. Schematic representation of the BacterioMatch two‐hybrid system. Plasmids pBT and pTRG are used to fuse prey proteins to λCI or replace the RNA polymerase α C‐terminal domain with target proteins. Upon expression in vivo, interactions between λCI‐prey and α‐bait fusions allow sufficient expression of His3 for growth in the presence of the His3 inhibitor 3‐AT.
B. BacterioMatch two‐hybrid system reporter cells were cotransformed with derivatives of the pBT and pTRG plasmids and vector‐only controls. YcgZ, YmgA, YmgB and YmgC were expressed either as hybrid proteins fused to cI‐NTD from pBT or as fusions to RNAP alpha‐NTD from pTRG. For experiments with RcsC or RcsD we cloned the entire cytosolic part of the protein or the indicated histidine kinase (RcsC‐HK), alpha‐beta‐loop (RcsC‐ABL) and phosphoreceiver domain (RcsC‐PR). Full‐size RcsB was expressed from pTRG. Interactions were detected by growth in the presence of the His3 inhibitor 3‐AT (selective) at 37°C for 24 h following incubation at 28°C for 48 h. Each row on the plates shows patches of five independent cotransformants.
C. The E. coli strain MC4100 encoding chromosomal ymgC::Flag, ymgA::Flag or ycgZ::Flag was transformed with pBAD33‐derivatives expressing His6‐RcsC, His6‐RcsB, or His6‐YmgA. YmgB‐Strep was expressed from pBAD18 and the empty vectors served as negative controls. Flag‐tagged proteins coeluting in Ni‐NTA or Strep‐Tactin Sepharose based chromatography of cellular lysates were detected using immunoblot analysis and the monoclonal anti‐Flag antibody.
D. Wild‐type MC4100 was cotransformed with pBAD18 or pBAD18‐ymgB‐Strep and either pBAD33‐His‐rcsC (cytosolic part) or pBAD33‐His‐rcsB. YmgB‐Strep coeluting with His‐RcsC or His‐RcsB from extracts subject to Ni‐NTA chromatography was detected using the anti‐Strep‐Tactin antibody. Lanes labelled (M) contain size markers. [Colour figure can be viewed at https://www.wileyonlinelibrary.com]
