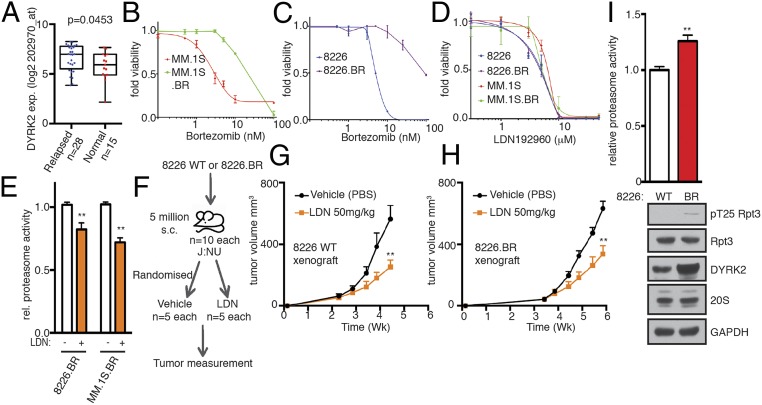
Fig. 6.
LDN192960 bypasses bortezomib resistance. (A) DYRK2 differential gene expression in human relapsed MM specimens and human normal tissue as available from a public database GSE6477 (relapsed vs. normal tissue, P value derived from empirical Bayes estimation on linear models of gene expression in limma package) (see also SI Appendix, Fig. S3). (B and C) Bortezomib EC50 for parental MM.1S and bortezomib-resistant MM.1S.BR cells (B) and for parental RPMI8226 and bortezomib-resistant 8226.BR cells (C). (D) LDN192960 EC50 for parental MM.1S, parental RPMI8226, MM.1S.BR, and 8226.BR cells. (E) Proteasome activity in total cell lysates from the indicated bortezomib-resistant cells with or without 10 μM LDN192960 treatment for 2 h was measured with Suc-LLVY-AMC and normalized to total protein content. **P < 0.01 (compared to control treated for each cell line, ordinary 1-way ANOVA, mean ± SD from n = 3 independent experiments). (F) Experimental flow for myeloma xenograft study in G and H. (G and H) The 8226 parental (G) or 8226.BR (H) cells were injected s.c. into J:NU nude mice. Palpable tumor-bearing mice were randomized (16 d for parental and 23 d for 8226.BR) into 2 equal groups each and treated with vehicle control or LDN192960 3 times a week by i.p. injection, and tumor volume was measured twice a week (n = 5 per condition). **P < 0.01 (compared to vehicle treated, 2-way ANOVA, mean ± SD, from n = 5 mice). (I) Proteasome activity in total cell lysates from 8226 WT or 8226.BR was measured with Suc-LLVY-AMC. **P < 0.01 (8226 WT vs. 8226.BR, unpaired Student’s t test, mean ± SD from n = 3 independent experiments). Immunoblotting of the cell lysates was carried out with indicated antibodies.