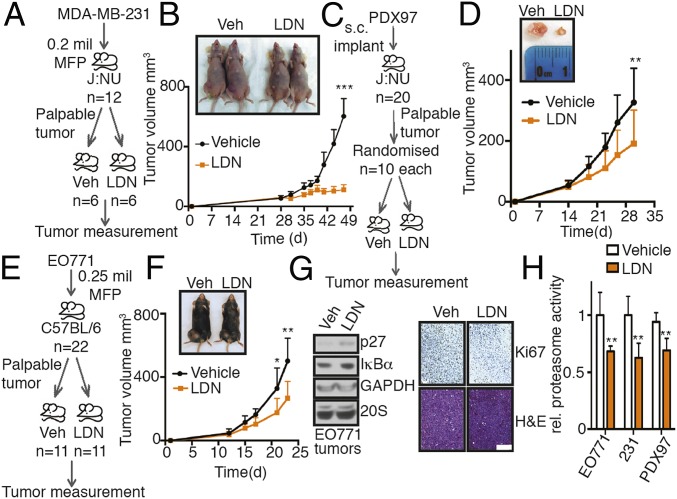
Fig. 7.
LDN192960 perturbs TNBC progression. (A) Experimental flow for TNBC xenograft study in B. (B) MDA-MB-231 parental cells were injected into the mammary fat pad of J:NU nude mice. Palpable tumor-bearing mice were randomized into 2 equal groups and treated with vehicle control or LDN192960 3 times a week by i.p. injection, and tumor volume was measured twice a week (n = 6 per condition). ***P < 0.001 (compared to vehicle treated, 2-way ANOVA, mean ± SD, from n = 6 mice). (C) Experimental flow for TNBC PDX study in D. (D) Patient-derived PDX97 tumor specimens were surgically implanted s.c. into J:NU mice. Palpable tumor-bearing mice were randomized into 2 equal groups and were treated with vehicle control or LDN192960 as in B (n = 10 per condition). **P < 0.01 (compared to vehicle treated, 2-way ANOVA, mean ± SD, from n = 10 mice). (E) Experimental flow for TNBC allograft study in F. (F) EO771 parental cells were injected into the mammary fat pad of C57BL/6 immunocompetent mice. Palpable tumor-bearing mice were randomized into 2 equal groups and were treated with vehicle control or LDN192960 as in B (n = 11 per condition). **P < 0.01, *P < 0.05 (compared to vehicle treated, 2-way ANOVA, mean ± SD, from n = 11 mice). (G) Immunoblotting with indicated antibodies in tumor lysates from F and histological examination of consecutive sections of the fixed tumors from F with Ki67 and hematoxylin/eosin staining were carried out. (Scale bar, 100 μm.) (H) Proteasome activity in whole tumor lysates from vehicle or LDN192960-treated tumor-bearing mice from B, D, and F were measured with Suc-LLVY-AMC. **P < 0.01 (compared to control treated, 2-way ANOVA, mean ± SD from n = 3 different tumors each).