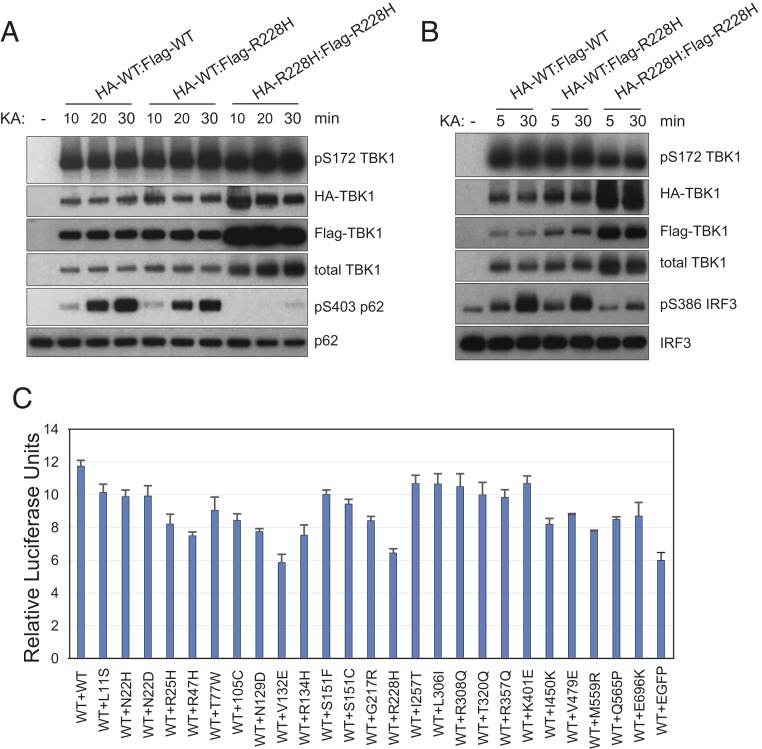
Fig. 4.
WT:mutant heterodimer TBK1 is functional. (A and B) WT:R228H heterodimer retains kinase activity toward p62 and IRF3. HA-TBK1-WT or HA-TBK1-R228H constructs were coexpressed with Flag-TBK1-WT or Flag-TBK1-R228H in TBK1 knockout 293T cells. Cell lysates were prepared 24 h after transfection and subjected to anti-HA beads immunoprecipitation for 2 h at 4 °C. After extensive washes, immunoprecipitated samples were eluted with HA peptide (0.5 μg/μL in wash buffer) and subjected to anti-Flag beads IP overnight. Final immunoprecipitated samples were assayed for kinase activity with recombinant GST-p62 (A) or Flag-IRF3 (B) on beads for increasing time (10, 20, and 30 min in A and 5 and 30 min in B). The phosphorylation status of various proteins was determined by western blots probed with specific antibodies. KA, kinase assay. (C) TBK1 ALS mutations do not inhibit WT TBK1 activity in IFNβ-luciferase reporter assays. Expression constructs for various TBK1 mutations were cotransfected with the same amount of WT expression construct plus IFNβ promoter-driven firefly luciferase construct and a renilla luciferase reference construct into TBK1 knockout 293T cells; luciferase activities were measured 24 h after transfection.